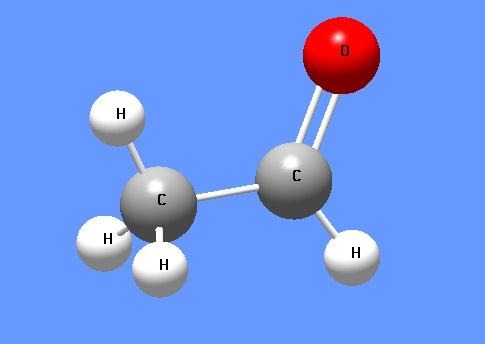
Acetaldehyde-Hazard and Toxicity
Description
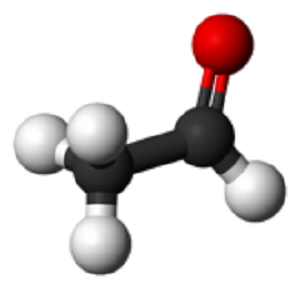
Acetaldehyde is an organic chemical compound with the formula CH3CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit,and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption.
Toxicity Data
LD50 oral (rat) 661 mg/kg
LC50 inhal (rat) 20,550 ppm (37,000 mg/m3; 30 min)
PEL (OSHA) 200 ppm (360 mg/m3)
TLV-TWA (ACGIH) 100 ppm (180 mg/m3)
STEL (ACGIH) 150 ppm (270 mg/m3)
Major Hazards
Highly flammable liquid; irritating to the eyes and respiratory system.
Toxicity
The acute toxicity of acetaldehyde is low by inhalation and moderate by ingestion.Exposure to acetaldehyde by inhalation is irritating to the respiratory tract and mucous membranes; this substance is a narcotic and can cause central nervous system depression. Ingestion of acetaldehyde may cause severe irritation of the digestive tract leading to nausea, vomiting, headache, and liver damage.
Acetaldehyde causes irritation and burning upon skin contact and is a severe eye irritant.
Acetaldehyde has caused nasal tumors in rats exposed by inhalation and is listed by IARC in Group 2B ("possible human carcinogen"). It is not classified as a "select carcinogen" according to the criteria of the OSHA Laboratory Standard.Acetaldehyde is mutagenic and has been shown to be a reproductive toxin in animals. Acetaldehyde is formed by metabolism of ethanol, and chronic exposure can produce symptoms similar to alcoholism.
Flammability and Explosibility
Acetaldehyde is a dangerous fire hazard (NFPA rating = 4) owing to its volatility and low autoignition temperature. Its vapor is explosive in the concentration range 4 to 66% in air and may be ignited by hot surfaces such as hot plates or light bulbs, or by static electricity discharges. The vapor is heavier than air and may travel a considerable distance to an ignition source and "flash back." Carbon dioxide or dry chemical extinguishers should be used to fight acetaldehyde fires.Reactivity and Incompatibility Acetaldehyde is a reactive substance and on storage in the presence of air may undergo oxidation to form explosive peroxides. It may also polymerize violently .when in contact with strong acids or trace metals such as iron. Acetaldehyde may undergo violent reactions with acid chlorides, anhydrides, amines, hydrogen cyanide,and hydrogen sulfide.
Reactivity and Incompatibility
Acetaldehyde is a reactive substance and on storage in the presence of air may undergo oxidation to form explosive peroxides. It may also polymerize violently when in contact with strong acids or trace metals such as iron. Acetaldehyde may undergo violent reactions with acid chlorides, anhydrides, amines, hydrogen cyanide,and hydrogen sulfide.
Storage and Handling
Acetaldehyde should be handled in the laboratory using the "basic prudent practices"described in Chapter 5.C, supplemented by the additional precautions for dealing with extremely flammable substances . In particular, acetaldehyde should be used only in areas free of ignition sources, and quantities greater than 1 liter should be stored in tightly sealed metal containers in areas separate from oxidizers. Acetaldehyde should always be stored under an inert atmosphere of nitrogen or argon to prevent autoxidation.
Accidents
In the event of skin contact, immediately wash with soap and water and remove contaminated clothing. In case of eye contact, promptly wash with copious amounts of water for 15 min (lifting upper and lower lids occasionally) and obtain medical attention. If acetaldehyde is ingested, obtain medical attention immediately. If large amounts of this compound are inhaled, move the person to fresh air and seek medical
attention at once.
In the event of a spill, remove all ignition sources, soak up the acetaldehyde with a spill pillow or absorbent material, place in an appropriate container, and dispose of properly. Alternatively, acetaldehyde spills may be neutralized with sodium bisulfite solution before cleanup. Respiratory protection may be necessary in the event of a
large spill or release in a confined area.
Disposal
Excess acetaldehyde and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.
);You may like
Related articles And Qustion
Lastest Price from Acetaldehyde manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 75-07-0
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $10.00/KG2024-01-08
- CAS:
- 75-07-0
- Min. Order:
- 10KG
- Purity:
- 99.50%
- Supply Ability:
- 1000kg