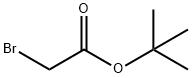
About tert-Butyl bromoacetate
Abstract
The invention relates to a synthesis method of tert-butyl bromoacetate, and the synthesis method comprises the following steps of: mixing bromoacetic acid and tert-butyl acetate at a weight ratio of 4:(1-1):1, and completely dissolving bromoacetic acid in tert-butyl acetate; then, feeding the solution into a reactor filled with strong acid type ion exchange resin, controlling the temperature within the range of 30-45 DEG C, reacting for 2-6 hours, and generating a mixture with 30-35% tert-butyl bromoacetate; and separating by two rectifying towers, thus obtaining the tert-butyl bromoacetate with the purity of more than 99%, wherein more than 90% of tert-butyl acetate is completely reacted, and the effective transformation rate of the tert-butyl bromoacetate is more than 80%. According to the invention, the original technology is changed, a two-step production method is replaced by a one-step production method, and the tert-butyl acetate is used for replacing the original thionyl chloride with strong corrosivity and irritation and taken as a raw material, so that the three wastes are not produced in the production process, and the synthesis method is in accordance with the environmental protection policy of China.
Background technique
[0002] t-butyl bromoacetate is an important pharmaceutical intermediates, the production method is mainly used at home and abroad, "Facile Synthesis of tert-butyl bromide method" described in the literature, which is a two-step method, the main process described as follows:
Thionyl chloride, bromoacetic acid + a bromoacetyl chloride bromoacetyl chloride + t-butoxide a t-butyl acetate bromine chloride +
Laboratory data in this reaction, the excess thionyl chloride, bromoacetyl chloride in 95% yield, the process has to use atmospheric distillation, vacuum distillation, obtained by reacting bromoacetyl chloride, bromoacetyl chloride and then t butoxide, sodium tripolyphosphate as the acid-binding agent, and reaction with the addition of chloroform, the reaction product was washed with inorganic salts, dried, and distilled at atmospheric pressure and distilled under reduced pressure to give tert-butyl bromoacetate and finally, the yield 93% two-step, two-step total yield laboratory was 89%, a total process is complicated, and a large number of waste generated, environmental pollution, high cost, not suitable scale, continuous production.
SUMMARY
[0003] The present invention is directed to the problems in the prior art, using a new raw material which is to replace part of the reaction, changes from the original one-step two-step process, recycled material generated in the production process can be effective recycling, no waste production, and can implement large-scale, continuous production, help reduce production costs.
[0004] The present invention is reacted with tert-butyl bromoacetate.
[0005] The chemical formula of the present invention are:
BrCH2COOH + C6H12O2 - C6H11BrO2 + CH3COOH
Process of the present invention is: a weight ratio of 4: 1 to I: 1, bromoacetic acid (solid at room temperature) and tert-butyl acetate in the case where sufficient mixing of 40-55 ° C, using t-butyl acetate all bromoacetic acid was dissolved, and then passed through a reactor equipped with a cation exchange resin catalyst, the control temperature cycling reaction in the range of 35-45 ° C, 2-6 hours after the reaction cycle, generating containing t-butyl bromoacetate content of 20-45% of the mixture. The resulting reaction product was sent to the separation section for distillation, first through a column debromination acid, bromoacetic acid isolated distillation under reduced pressure and a small amount of high boiling point substances C12, bromoacetic acid recovered again participate in the reaction, and then purified by column , acetic acid splitter and tert-butyl bromoacetate remaining low boiling point, tert-butyl acetate, bis isobutene, taken out from the bottom of t-butyl bromoacetate in a purity of 99% or more, a low boiling point material may be mixed as by-products for export, also available for the production of acetic acid t-butyl ester starting material.
[0006] with tert-butyl bromoacetate in the present invention, preferably the weight ratio of 3: 1.
[0007] In the present invention, equipped with a cation exchange resin catalyst circulating reaction time is preferably 3-5 hours, most appropriate time was 4 hours.
[0008] In the present invention, a strong acid type ion exchange resin catalyst a strongly acidic macroporous ion catalyzed reaction of the resin, for example: the Hebei Chemical Co. Kerry KC117, KC118 type catalyst equivalent.
The reaction product of the present invention, by chromatographic analysis, more than 90% of t-butyl acetate completion of the reaction, wherein the effective conversion of tert-butyl bromoacetate to generate more than 80%, the reaction product, which is the main constituent (the content thereof t-butyl bromoacetate; acetate, 8-16%; acetate, 2-8%; C8 (double isobutylene), I 5-4 5%; bromoacetic acid, 35-65%: weight percent). , 20-45%;.. C12,0 1-0 9%.
[0009] Advantageous Effects of Invention:
1, completely changed the original process, by the two-step production process into a one-step production, and have implement large-scale, continuous production conditions conducive to a significant decline in the cost of production;
2, the production process is relatively simple, easy operation control;
3, production processes greener, more environmentally friendly, eliminating corrosive, strong irritant material thionyl chloride, in a friendly environment acetate solvent as starting materials for substitution reactions, and the production process generates substantially no waste, in line with national environmental policy; 4, the reaction process is more moderate, low-risk production.
);You may like
Related articles And Qustion
See also
Lastest Price from tert-Butyl bromoacetate manufacturers

US $5.00/KG2024-04-19
- CAS:
- 5292-43-3
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 500mt/year

US $0.00/kg2023-12-11
- CAS:
- 5292-43-3
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20tons