4-Chloro-7H-pyrrolo[2,3-d] pyrimidine— a medical intermediate
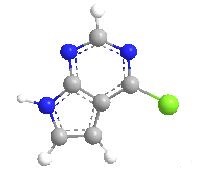
Being an important intermediate in medicine, 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine had been widely used in the synthesis of many pharmaceutical intermediates at home and abroad. Including CP690550, CGP76030 and so on, 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine was the scaffold for many commercially available drugs. Also, 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine was the intermediate of the bulk drug, Tofatinib, which was widely used in treatment of adult patients with moderate to severe rheumatoid arthritis with inadequate or intolerant methotrexate response.
Preparation method for 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine: Ethyl cyantoacetate and thiourea were dissolved in ethanol at a molar ratio of 1:1~1:3, and sodium ethoxide, equal to 1.5~3 times of the molar amount of ethyl cyantoacetate, was slowly added at 0~5℃. The mixture was stirred at room temperature for 1~2 hours, then heated to 80~100℃, refluxed for 8~10 hours. Afterwards, the reaction was cooled to room temperature, filtered, washed with solid ethanol, and dried to obtain 2-mercapto-4-amino-6-hydroxypyrimidine. Then, the obtained 2-mercapto-4-amino-6-hydroxy pyrimidine was added to the ammonia, amount of which was equal to 10~20 times of the weight of 2-mercapto-4-amino-6-hydroxy pyrimidine. Subsequently, 3 ~ 5 times of the molar volume of active nickel was added. Then, the reaction system was heated to 80 ~ 100 ℃, refluxed for 4 ~ 6 hours. With immediate filtration, active nickel could be removed, and then the reaction was cooled to room temperature. During this course, solid was separated out. Following filtration and washing with water, 4-amino-6-hydroxy pyrimidine would be obtained with drying. Next, 4-amino-6-hydroxy pyrimidine and sodium acetate were dissolved in water with the molar ratio of 1:3 ~ 5. Heated to 60 ~ 80 ℃, solution of 2-chloroacetaldehyde was added slowly. By the way, the dosage of 2-chloroacetaldehyde was equal to 2 ~ 5 times of molar volume of 4-amino-6-hydroxy pyrimidine. After stirring for 4 ~ 6 hours, the reaction was cooled to room temperature and filtered. Washing with water, the solid was dried to give 4-hydroxypyrropyrimidine.
Then, it was dissolved into phosphorus oxychloride, stirred at 80~100℃ for 2~4 hours, distilled to remove the excess phosphorus oxychloride. In the next, the reaction was cooled to 0~10℃, quenched with ice water, stirred for 20~30 minutes. After that, the pH of the solution was adjusted to 9~10 with sodium hydroxide solution. Then, with filtration and washing with water, crude product of 4-chloropyrrole pyrimidine was obtained. Recrystallized with toluene, pure 4-chloropyrrole pyrimidine would be obtained.
References
[1]. 陈林,张艳 一种4-氯吡咯并嘧啶的制备方法: 中国, ZL201010174595.4[P]. 2012.
);You may like
Related articles And Qustion
See also
Lastest Price from 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine manufacturers
![3680-69-1 4-Chloropyrrolo[2,3-d]pyrimidine](https://img.chemicalbook.com/ProductImageEN/2019-3/Small/f0c7e8e7-13cd-4ffd-9857-c465e8e31b7f.jpg)
US $22.00-20.00/KG2024-04-23
- CAS:
- 3680-69-1
- Min. Order:
- 100 mkg
- Purity:
- 99%
- Supply Ability:
- 20mt
![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d]pyrimidine](/ProductImageEN/2024-04/Small/3c43703f-a82c-455f-8c7b-e8a31b1b6156.gif)
US $5.00-0.10/KG2024-04-05
- CAS:
- 3680-69-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- g-kg-tons, free sample is available
![3680-69-1 4-Chloro-7H-pyrrolo[2,3-d] pyrimidine;preparation; synthesis;intermediate](https://www.chemicalbook.com/CAS/GIF/3680-69-1.gif)
