3,4-Dimethylaniline-Application
3,4-dimethylaniline is a primary arylamine that is aniline in which the hydrogens at the 3- and 4-positions are replaced by methyl groups. A low-melting, crystalline solid, 3,4-dimethylaniline is used in the production of vitamin B2, dyes, pesticides and other chemicals. 3,4-dimethylaniline is a dimethylaniline and a primary arylamine. 3,4-dimethylaniline appears as pale brown crystals or off-white solid[1].
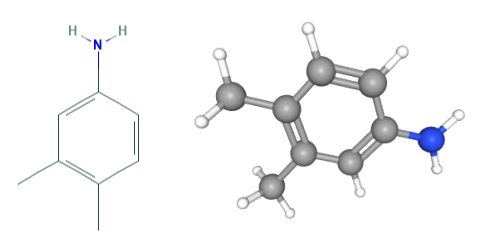
Fig 1. Chemical structure formula and three-dimensional structure of 3,4-Dimethylaniline
3,4-dimethylaniline used as a chemical intermediate for the synthesis of riboflavin. Soboley and Berezooskii patented the production of 3,4-dimethylaniline by the hydrogenation of 2-chloromethyl-4-nitrotoluene[2].
Symptoms of exposure to 3,4-dimethylaniline may include headaches, drowsiness, cyanosis, mental confusion, convulsions, anorexia, nervous system and blood effects, fatigue, loss of appetite, dizziness, and damage to the lungs, liver and kidneys. 3,4-dimethylaniline may be absorbed through the skin and may cause irritation on contact. When heated to decomposition it emits toxic fumes[3].
The major hazards encountered in the use and handling of 3,4-dimethylaniline stem from its toxicologic properties. Toxic by all routes (ie, inhalation, ingestion, and dermal absorption), exposure to this crystalline material may occur from its use as a chemical intermediate for the synthesis of riboflavin (vitamin B2).
Contact with 3,4-dimethylaniline may cause burns to the skin and eyes. Death is also a possible outcome from exposure. While the OSHA PEL is set at a TWA of 5 ppm, the ACGIH lists xylidine (mixed isomers) on its 1988-1989 Notice of Intended Changes with a proposed TLV of 0.5 ppm, and a designation of "suspected human carcinogen". In activities or situations where over-exposure may occur, wear a self-contained breathing apparatus, and full chemical protective clothing which is specifically recommended by the shipper or manufacturer to prevent skin contact with xylidine. If contact should occur, immediately flush the affected skin or eyes with running water for at least 15 minutes. Remove contaminated clothing and shoes at the site. Do not eat or smoke in xylidine work areas. While xylidine does not ignite easily, it can burn with the production of irritating or poisonous gases.
Also, containers may explode violently in the heat of a fire. Fires involving xylidine may be extinguished with dry chemical, CO2, Halon, or standard foam. Water spray if used, should be applied with caution because it may cause frothing. Fight the fire from a maximum distance and dike runoff from fire control water. Xylidine should be stored away from heat, strong oxidizers, and hypochlorite bleaches. Shipping regulations and other DOT regulatory requirements should be consulted before transport. If 3,4-dimethylaniline should spill, carefully shovel the material into clean covered containers (solutions are first diked far ahead, then taken up with sand or other noncombustible absorbent) for later disposal. Incineration is a possible form of disposal for xylidine. Before implementing land disposal of waste 3,4-dimethylaniline, consult with environmental regulatory agencies for guidance.
3,4-dimethylaniline ignites on contact with fuming nitric acid. Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides[4].
References
[1]Ashford, R.D. Ashford's Dictionary of Industrial Chemicals. London, England: Wavelength Publications Ltd., 1994. p. 958.
[2]Kirk-Othmer Encyclopedia of Chemical Technology. 3rd ed., Volumes 1-26. New York, NY: John Wiley and Sons, 1978-1984., p. V2 365 (1978).
[3]National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina.
[4]R. L. MELNICK, J. E. HUFF, J. K. HASEMAN. Chronic Toxkity Results and Ongoing Studies of 1,3-Butadiene by the National Toxicology Program [J]. Annals of the New York Academy of Sciences, 2006, 534(Living in a Chemical World: Occupational and Environmental Significance of Industrial Carcinogens):648-662.
);You may like
See also
Lastest Price from 3,4-Dimethylaniline manufacturers

US $9.00/KG2023-07-14
- CAS:
- 95-64-7
- Min. Order:
- 1KG
- Purity:
- 99.9
- Supply Ability:
- 1 ton

US $7.00/KG2019-09-02
- CAS:
- 95-64-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- JD 594