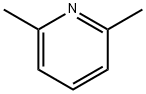
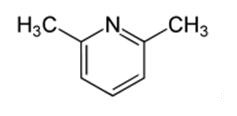
2,6-Lutidine-an aromatic organic compound
2,6-Lutidine is a natural heterocyclic aromatic organic compound with the formula (CH3)2C5H3N. It is one of several dimethyl-substituted derivatives of pyridine. It is a colorless oily liquid with mildly basic properties and a pungent, noxious odor of pyridine and peppermint. The boiling point is 144.0 °C/760 mmHg, 79 °C/87 mmHg. The melting point is -5.9 °C. The density is 0.9252 g/cm3 (20 °C). The major synthetic applications reported for 2,6-lutidine exploit its weakly nucleophilic nature (a result of steric crowding at the ring nitrogen) but moderately basic character (pKa of its conjugate acid is 6.7). 2,6-Lutidine is soluble in common organic solvents (e.g. ether, THF, DMF, alcohol) and shows considerable water solubility (ca. 27% (w/w) at 45 °C) [1].
2,6-Lutidine was first isolated from the basic fraction of coal tar and from bone oil. A laboratory route involves condensation of ethyl acetoacetate, formaldehyde, and an ammonia source to give a bis(carboxy ester) of a 2,6-dimethyl-1,4-dihydropyridine, which, after hydrolysis, undergoes decarboxylation. It is produced industrially by the reaction of formaldehyde, acetaldehyde, and ammonia [2].
2,6-Lutidine has been evaluated for use as a food additive owing to its nutty aroma when present in solution at very low concentrations.
Due to the steric effects of the two methyl groups, 2,6-lutidine is only weakly nucleophilic. Protonation of lutidine gives lutidinium, [(CH3)2C5H3NH]+, salts of which are sometimes used as a weak acid because the conjugate base (2,6-lutidine) is so weakly coordinating. In a similar implementation, 2,6-lutidine is thus sometimes used in organic synthesis as a sterically hindered mild base [3].
2,6-Lutidine has been identified as a beneficial additive for the oxidative cleavage of olefins to ketones by NaIO4 in the presence of catalytic RuCl3, improving the yield and shortening the reaction times. In the absence of 2,6-lutidine reactions stalled at the diol intermediate with incomplete conversion to the desired ketones. The reaction protocol described herein also avoids the use of harmful solvents such as CCl4 and DCE and is tolerant of a range of functional groups.
The biodegradation of pyridines proceeds via multiple pathways [4]. Although pyridine is an excellent source of carbon, nitrogen, and energy for certain microorganisms, methylation significantly retards degradation of the pyridine ring. In soil, 2,6-lutidine is significantly more resistant to microbiological degradation than any of the picoline isomers or 2,4-lutidine. Estimated time for complete degradation was >30 days [5].
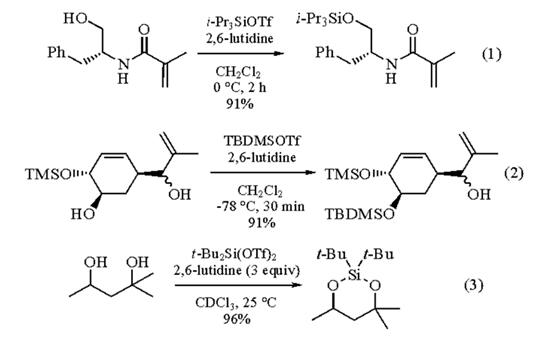
2,6-Lutidine is used in alcohol protection reactions [6]:
The protection of alcohols as hindered silyl ethers proceeds in >90% yield using the appropriate trialkylsilyl triflate in the presence of anhydrous 2,6-lutidine as an acid scavenger.
Triisopropylsilyl Trifluoromethanesulfonate/2,6-lutidine in CH2Cl2 (-78 °C to 0 °C) allows for the protection of primary and secondary (but not tertiary) alcohols (eq 1). Tertiary alcohols can be protected as the somewhat less crowded i-PrEt2Si ether (i-PrEt2SiOTf/2,6-lutidine, CH2Cl2, rt). The silylation of tertiary and unreactive secondary alcohols can also be achieved using t-Butyldimethylsilyl Trifluoromethanesulfonate/2,6-lutidine in CH2Cl2 (0-25 °C); silylation at -78 °C allows for the selective protection of a secondary alcohol in the presence of a secondary allylic alcohol (eq 2). The silylation of secondary allylic alcohols as the bulky tribenzylsilyl ether or tri-p-xylylsilyl ether can be accomplished using the appropriate tris(arylmethyl)chlorosilane/2,6-lutidine in DMF at -20 °C. The bis-protection of 1,2-, 1,3-, and 1,4-diols as the corresponding dialkylsilylene derivatives can be achieved in high yield using Diisopropylsilyl Bis(trifluoromethanesulfonate) or Di-t-butylsilyl Bis(trifluoromethanesulfonate) at rt in CDCl3 in the presence of 2,6-lutidine (eq 3); interestingly, 2,6-lutidine was found to retard the 3,5-protection of nucleosides in DMF solution. Protection of a 1,3-diol as its methylene acetal can be achieved using Trimethylsilyl Trifluoromethanesulfonate/2,6-lutidine in MeOCH2OMe (0 °C, 15 min, 79%).
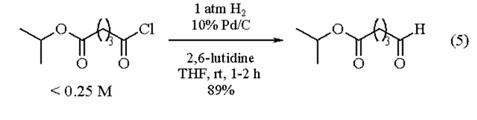
Other Protection/Deprotection Reactions.
2,6-Lutidine is more effective than Silver(I) Carbonate or other amine bases at mediating the conversion of a 2-acetylpyranosyl bromide to an orthoester (eq 4). Side reactions are minimized due to the lower acidity of the conjugate acid of 2,6-lutidine (pKa 6.7) when compared to the other substituted pyridines examined. Exo stereoselectivity is maximized when using 2,6-lutidine rather than silver carbonate. 2,6-Lutidine considerably accelerates bond cleavage during the triarylamine radical cation-mediated oxidative cleavage of primary and secondary benzyl and p-methoxybenzyl ethers to the corresponding alcohols.
References
[1] https://en.wikipedia.org/wiki/2,6-Lutidine
[2] Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2007). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_399.
[3]Prudhomme, Daniel R.; Park, Minnie; Wang, Zhiwei; Buck, Jason R.; Rizzo, Carmelo J. (2000). "Synthesis of 2'-Deoxyribonucleosides: Β-3',5'-Di-o-benzoylthymidine". Org. Synth. 77: 162. doi:10.15227/orgsyn.077.0162.
[4]Philipp, Bodo; Hoff, Malte; Germa, Florence; Schink, Bernhard; Beimborn, Dieter; Mersch-Sundermann, Volker (2007). "Biochemical Interpretation of Quantitative Structure-Activity Relationships (QSAR) for Biodegradation of N-Heterocycles: A Complementary Approach to Predict Biodegradability". Environmental Science & Technology. 41: 1390-1398. doi:10.1021/es061505d.
[5] Sims, G. K.; Sommers, L.E. (1986). "Biodegradation of Pyridine Derivatives in Soil Suspensions". Environmental Toxicology and Chemistry: 503–509. doi:10.1002/etc.5620050601.
[6] http://reag.paperplane.io/00001773.htm
You may like
Related articles And Qustion
See also
Lastest Price from 2,6-Lutidine manufacturers

US $0.00/KG2023-06-29
- CAS:
- 108-48-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month

US $10.00/KG2023-01-10
- CAS:
- 108-48-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt