1,3-Bis(diphenylphosphino)propane-An Organophosphorus Compound
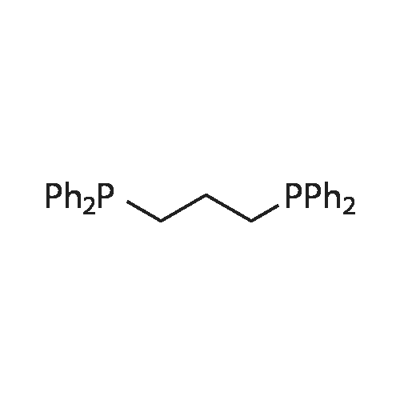
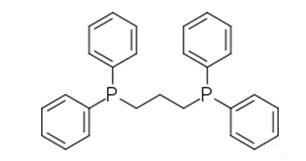
1,3-Bis(diphenylphosphino)propane (DPPP) is an organophosphorus compound with the formula Ph2P(CH2)3PPh2. The compound is a white solid that is soluble in organic solvents. It is insoluble in water. It is slightly air-sensitive, degrading in air to the phosphine oxide. It is classified as a diphosphine ligand in coordination chemistry and homogeneous catalysis. It should be stored in a cool, dry place and in a closed container.
The diphosphine can be prepared by the reaction of lithium diphenylphosphide and 1,3-dichloropropane (Ph = C6H5):
2Ph2PLi + C3H6Cl2 → Ph2P(CH2)3PPh2 + 2LiC
However, it can be synthesised via a much more controllable (and cheaper) route, via metal-halogen exchange and then metathesis:
Br(CH2)3Br + 2 tBuLi→Li(CH2)3Li + 2 tBuBr
Li(CH2)3Li + 2 PCl3→Cl2P(CH2)3PCl2 + 2 LiCl
Cl2P(CH2)3PCl2 + 4 PhLi→Ph2P(CH2)3PPh2 + 4 LiCl
1,3-Bis(diphenylphosphino)propane is used in coordination chemistry and used as co-catalyst. The diphosphine serves as a bidentate ligand forming six-membered C3P2M chelate ring with a natural bite angle of 91o [1]. For example, the complex dichloro (1,3-bis(diphenylphosphino)propane) nickel is prepared by combining equimolar portions of the ligand and nickel (II) chloride hexahydrate. This nickel complex serves as a catalyst for the Kumada coupling reaction [2]. DPPP is also used as a ligand for palladium (II) catalysts to co-polymerize carbon monoxide and ethylene to give polyketones [3]. DPPP can sometimes be used in palladium-catalyzed arylation under Heck reaction conditions to control regioselectivity [4].
DPPP is used to complex a divalent palladium catalyst to carry out copolymerization of carbon monoxide and ethylene to obtain a polyketone.
The silver(I) coordination polymers have attracted great interest not only for their extensive applications as functional materials, but also for their intriguing architectures. Because of their potential applications in the fields of biological activity, catalysis and light emitting devices, the mixed IB metal complexes combining bisphosphines (PP) and aromatic diamine ligands have been intensively investigated during past years. The reactions of 1,3-bis(diphenylphosphino)propane and 1,10-phenanthroline derivative with Ag salts in the mixed solvent of methanol and dichloromethane generate the corresponding complexes, {[Ag2(dppp)2(phen)2](BF4)2(H2O)2}n, {[Ag2(dppp)2-(dmp)2](CF3SO3)2}n, {[Ag2(dppp)2(dmp)2](BF4)}n (dmp = neocuproine), {[Ag2(dppp)2(Bphen)2](CF3SO3)2}n and {[Ag2(dppp)2(Bphen)2](ClO4)} (Bphen = 4,7-diphenyl-1,10-diazaphenanthrene) [6].
Binuclear Ni(II) complexes of 4-diethylaminosalicylaldehyde-4(N)-substituted thiosemicarbazones can be bridged by 1,3-bis(diphenylphosphino)propane.
Potential Health Effects of 1,3-Bis(diphenylphosphino)propane
Eye: Causes eye irritation.
Skin: Causes skin irritation. May be harmful if absorbed through the skin.
Ingestion: May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation: Causes respiratory tract irritation. May be harmful if inhaled.
Chronic: No information found.
References
[1] Birkholz (née Gensow), Mandy-Nicole; Freixa, Zoraida; van Leeuwen, Piet W. N. M. (2009). "Bite angle effects of diphosphines in C–C and C–X bond forming cross coupling reactions". Chemical Society Reviews. 38 (4): 1099. doi:10.1039/B806211K.
[2] Kumada, Makota; Tamao, Kohei; Sumitani, Koji (1988). "Phosphine-Nickel Complex Catalyzed Cross-Coupling of Grignard Reagents with Aryl and Alkenyl Halides: 1,2-dibutylbenzene". Organic Syntheses.; Collective Volume, 6, p. 407
[3] Drent, E.; Mul, W. P.; Smaardijk, A. A. (2001). "Polyketones". Encyclopedia Of Polymer Science and Technology. doi:10.1002/0471440264.pst273.
[4] Cabri, Walter; Candiani, Ilaria; Bedeschi Angelo; Penco, Sergio"α-Regioselectivity in Palladium-Catalyzed Arylation of Acyclic Enol Ethers" journal= Journal of Organic Chemistry, 1991, volume 57, p. 1481. doi:10.1021/jo00031a029
[5] https://en.wikipedia.org/wiki/1,3-Bis(diphenylphosphino)propane
[6] Yuan Yuan, Hong-Liang Han, Sen Lin, Yang-Zhe Cui, Min Liu, Zhong-Feng Li, Qiong-Hua Jin, Yu-Ping Yang, Zhen-Wei Zhang, Synthesis, structural characterization, stability, antibacterial activity and spectroscopic properties (THz) of five new polynuclear silver(I) complexes with 1,10-phenanthroline derivative and 1,3-bis(diphenylphosphino)propane (dppp), Polyhedron 119 (2016) 184–193.
You may like
See also
Lastest Price from 1,3-Bis(diphenylphosphino)propane manufacturers
US $10.00-178.00/g2024-02-23
- CAS:
- 6737-42-4
- Min. Order:
- 25g
- Purity:
- 0.98
- Supply Ability:
- 100kg

US $30.00-1.00/kg2023-12-11
- CAS:
- 6737-42-4
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 10 tons