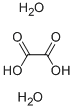
What is the relationship between oxalic acid and oxalic acid dihydrate?
Description
Oxalic acid is a relatively strong acid despite being a carboxylic acid. Oxalic acid has many applications in the laboratory as an analytical reagent and a general reducing agent. The concentration of oxalic acid in solution may be precisely determined by titration with potassium permanganate[1]. Oxalic acid has two forms. One is only oxalic acid, and the other has crystallization watern. The one with no crystallization water is called oxalic acid or anhydrous oxalic acid. On the other hand, the one with two waters of crystallization is called oxalic acid dihydrate. Solid oxalic acid dihydrate can be dehydrated with heat or azeotropic distillation.

Oxalic acid dihydrate
Oxalic acid dihydrate is a promising phase change material that may be useful for thermal energy storage. Oxalic acid dihydrate is monoclinic with space group P21/n. The calculated lattice parameters, volume, and density are given in Figure 1, where they are compared with the experimental values given by various authors at several temperatures. The computed crystal structure of oxalic acid dihydrate is shown in Figure 2, where views of the unit cell from [100], [001], and [001] directions are provided[2]. It is produced naturally by the metabolism of glycine and ascorbic inside the living bodies. Due to the polar nature of oxalic acid, it has received special attention, especially in electro-optical effects and second harmonic generation. Oxalic acid also has a wide range of applications in science, industry, and medicine, making it essential to study its physio-chemical properties in great detail.
The uses of oxalic acid
One of the most typical applications of oxalic acid is an actinometer used as a standard for measuring light energies involved in photochemical studies. Some esters of oxalic acid may be used in practical applications requiring organic chemiluminescent materials. Oxalic acid has been observed as a degradation product in the bitumen used to encapsulate radioactive waste resulting from reprocessing spent nuclear fuel exposed to gamma rays. Oxalic acid may increase the leaching of radionuclides conditioned in bitumen for radioactive waste disposal. Oxalic acid, under natural conditions, is not toxic but becomes so because of the formation of oxalateions. The excessive ingestion of oxalic acid or prolonged skin contact with this compound can be dangerous. This compound is also widely used as an acid rinse in laundries. It removes rust and ink stains by converting most insoluble iron compounds into soluble complex ions. For the same reason, it is the main constituent of many preparations for removing scale from automobile radiators. Oxalic acid is also a bleaching agent and a fixative dye substance.
References
[1] K. Mahendra . “Systematic investigation and comparison of oxalic acid dihydrate, ammonium oxalate hydrate and ammonium hydrogen oxalate oxalic acid dihydrate single crystals.” Materials Science and Engineering B-advanced Functional Solid-state Materials 288 (2023): Article 116157.
[2] Colmenero, Francisco. “Mechanical properties of anhydrous oxalic acid and oxalic acid dihydrate†.” Physical Chemistry Chemical Physics 5 (2019): 2673–2690.
);You may like
See also
Lastest Price from Oxalic acid dihydrate manufacturers

US $5.00/KG2023-10-25
- CAS:
- 6153-56-6
- Min. Order:
- 200000KG
- Purity:
- 99.6% MIN
- Supply Ability:
- 100000MT

US $5.00/KG2023-10-25
- CAS:
- 6153-56-6
- Min. Order:
- 200000KG
- Purity:
- 99.6% MIN
- Supply Ability:
- 100000MT