What is the crystal structure of Beryllium carbide?
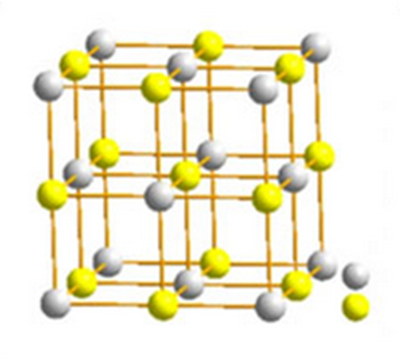
Beryllium carbide in its pure form is a colorless transparent crystal historically identified by Lebeau with Be2C composition. Be2C is Fluorite structured and crystallizes in the cubic Fm-3m space group. Recently, some additional possible structures of beryllium carbide have been identified to be energetically stable, including quasi planar hexacoordinate carbon, a promising 2D material, or a stable hexa-coordinate monolayer. A wide range of applications for beryllium carbide such as for ablation coatings in experimental fusion reactors or as the diffusion barrier against atomic copper has recently drew attention to this material.
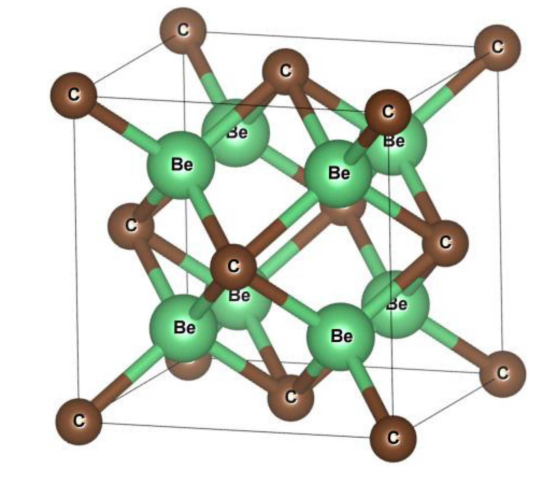
Beryllium carbide is used in inertial confinement fusion (ICF) capsule ablation material due to its low atomic number, low opacity, and high melting point properties. The diffusion barrier of copper atom in crystal beryllium is only 0.79 eV, and the barrier in beryllium carbide is larger than 2.85 eV. The three structures of beryllium carbide: anti-fluorite Be2C, Be2C-I, and Be2C-III have a good blocking effect to the diffusion of copper atom. Among them, the Be2C-III structure has the highest diffusion barrier of 6.09 eV.
Using electron localization function and the Bader charge analysis we also determined a bonding character inside Be2C. By utilizing the electron localization function, the nature of bonding was specified. The bonding between carbon and beryllium is of ionic nature, while the bonding between the nearest Be has a covalent character. The Bader charge analysis seems to confirm this observation, showing the additional charge accumulated at carbon ions while the charge is depleted of beryllium.
Reference:
[1] Gruszka, K. “First Principle Study of Structural and Electronic Properties of CBe2 Compound.” Acta Physica Polonica A 13 1 (2021).
);