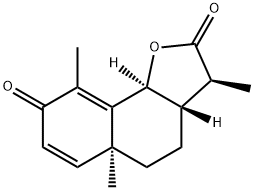
What is Santonin?
Description
Santonin is a white solid consisting of colorless flat prisms, turning slightly yellow from the action of light and soluble in alcohol, chloroform, and boiling water. It exists in two forms, α-santonin and β-santonin.
As an anthelmintic
Santonin was first isolated from unexpanded flower heads of Artemesia plants (Levant Wormseed) grown in Russian and Chinese Turkestan and the Southern Ural region[1]. The structure of this compound was established by Clemo et al. and Ruzicka and Steiner. Studies have found that santonin does not kill roundworms, but paralyzes parasitic worms (worms) and causes them to be excreted. Santonin could paralyze the anterior end of the worm and have a stimulating effect on the posterior end, depending on the concentration. As a result, worms are unable to self-coordinate and lose the ability to hold positions in the host. Subsequent use of laxatives allows the worms to be easily excreted[2].
Side effects
However, it has more serious side effects. It may cause gastric pain, pallor and coldness of the surface, followed by heat and injection of the head, tremors, dizziness, pupillary dilatation, twitching of the eyes, stertor, copious sweating, Hematuria, convulsive movements, tetanic cramps stupor, and insensibility. The toxicity of santonin can alter color vision and affect visual morphology, causing the user to have purple or yellow vision. As safer Ascaricides were developed, it has been replaced.
Photoinduced rearrangement
The rearrangement of santonin has been used by many researchers in the synthesis of a variety of natural products. Phil B. Alper and colleagues report novel photoinduced rearrangements of 2,5-dienones, including santonin. They found that the rearrangements can be “rerouted” in the presence of amines to give previously unknown structures[3]. Santonin derivatives such as 6-epi-β-santonin have also been utilized as starting material in the photochemical rearrangement. Santonin was aromatized with molecular iodine as the catalyst, and the resulting naproxen analogue has anti-inflammatory activity[4].
References
[1] Penati P, et al. Wormwood, nomads’ rights, and capitalism: the birth of a chemical industry in Russian Turkestan (1870s–1914). Modern Asian Studies, 2023; 57.
[2] Baldwin B. Modern Asian Studies. Parasitology, 2009; 35.
[3] Fisch M, et al.The Mechanism of the Photoconversion of Santonin. Journal of the American Chemical Society, 1963; 85: 3029–3030.
[4] Singh R, et al. Transformation of Santonin to a Naproxen Analogue with Anti-Inflammatory Activity, Journal of Natural Products, 2019; 82: 1710–1713.
Related articles And Qustion
See also
Lastest Price from Santonin manufacturers
US $15.00/g2022-07-06
- CAS:
- 481-06-1
- Min. Order:
- 1g
- Purity:
- ≥98%
- Supply Ability:
- 1000.00 kgs
US $1.10/g2021-06-19
- CAS:
- 481-06-1
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons Min