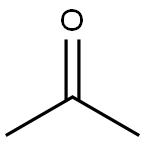
What is Acetone used for?
The appearance of acetone in scientific thinking may be traced back to 1798, when John Rollo, an English physician, described a material in human breath of an odor of decaying apples. Later this material was identified as acetone, and it was regarded as a characteristic feature of diabetic coma. By the turn of nineteenth and twentieth centuries, it became widely held that acetone was poorly, if at all, metabolized.
This picture started to
change from the end of 1940s since experimental data became
available showing the incorporation of 14C-carbons of labeled
acetone into cholesterol, fatty acids, urea, and glycogen. And
the oxidation of acetone to carbon dioxide exhaled in respiratory
air was also recognized. The possibility of in vivo formation
of glucose from acetone in experimental animals was also reported.
In 1980, the participation of cytochrome P450 type
enzymes in acetone breakdown was discovered. In 1984, the
pathways of acetone metabolism in rats were described.

Occurance
Acetone occurs naturally in plants, trees, volcanic gases, and
forest fires, and it is found in vehicle exhaust, tobacco smoke,
and landfill sites also. In the metabolic machinery of bacteria
and animals, acetone is also present. Industrial processes
contribute tonnages of acetone to the environment more than
natural processes.
As seen, a long-lasting standard dogma was that acetone was
an end product of metabolism in animals and humans.
Nonetheless, the pathways described in this article demonstrate
the potential for animals and humans to metabolize acetone.
Uses
Acetone has a characteristic odor of decaying apple and a sweetish taste. It is obtained by fermentation (retains a lesser proportion of the acetone market) or chemical synthesis (either as a main product or as a by-product) mainly via the cumene process to produce phenol. The uses of acetone in industry are many and based on its miscibility with water, alcohol, chloroform, ether, and most of the oils. Hence, its largest use is as a solvent in paint, varnishes, lacquers, plastics, and fibers, and a great variety of miscellaneous solvent uses (fats, oils). It is also used to make plastic, fibers, drugs, and other chemicals. In the laboratory, it is used to extract various substances from animal and plant tissues, and as a dehydrating agent. In housekeeping, it is found as a component of detergents and cosmetic products.
Mechanism of Toxicity
Acetone evaporates rapidly, even from water and soil. Once entered the atmosphere, it is degraded by photolysis, a reaction in which free radicals are involved or removed by wet deposits. It is a significant groundwater contaminant because of its miscibility in water.
Environmental Fate
In primates (including humans), isotretinoin (Accutane) is metabolized to a more active form, 13-cis-4-oxo-retinoic acid, which is able to move through the placental membrane. On its own, however, Accutane (isotretinoin) is not particularly motile across the placental barrier, and perhaps most interestingly tends not to bind to cellular retinoid-binding proteins or nuclear receptors. The rapid isomerization to the all-trans isomer, the oxidation of Accutane (isotretinoin) to 13-cis-4- oxo-retinoic acid, and the relatively high circulation times of these compounds may be important in explaining the teratogenic toxicity of Accutane (isotretinoin).
Some studies have more fully explored the metabolic products of isotretinoin. For example, isotretinoin can be metabolized in the liver by the cytochrome P450 microsomal enzyme system – more specifically the CYP2C8, CYP2C, CYP3A4, and CYP2B6 isoenzymes. The metabolites produced are numerous, including retinoic acid (tretinoin), 4-oxo-isotretinoin, and 4-oxo-retinoic acid (4-oxo-tretinoin). This relatively large array of retinoid metabolites may produce a variety of effects, most notably due to their higher potency as retinoids compared to the parent compound (isotretinoin).
It is possible that these additional metabolites are capable of binding to a variety of retinoid receptors in order to alter gene expression and further transcription or transrepression in protein synthesis, which may be responsible for the toxic effects of isotretinoin.
);