What is 2,2'-Dibromodiethyl ether?
Feb 11,2020
2,2'-dibromodiethyl ether is an important organic intermediate (building block) to synthetize substituted diethyl ether products.
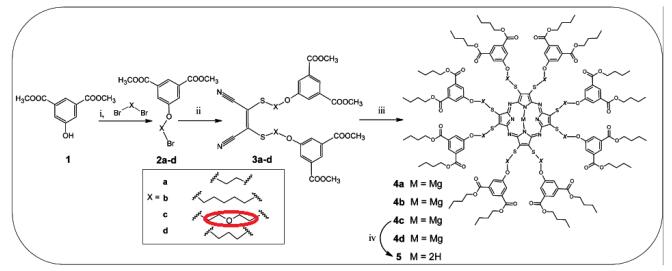
Gierszewski et al. reported [1] its application on the synthesis of porphyrazines with peripheral isophthaloxyalkylsulfanyl substituents. Peripheral sulfanyl substituents have helped to overcome many drawbacks related to a highly lipophilic porphyrazine system, especially its photochemical instability, poor solubility and/or aggregation in many solvents. Moreover, macrocycles of potential applications in photodynamic diagnosis (PDD) and photodynamic therapy (PDT) possess the following properties: (i) relatively long triplet state lifetimes (more than 1ms), (ii) relatively high values in intersystem-crossing quantum yields and (iii) singlet oxygen should be generated by the photosensitization process in the presence of porphyrinoid derivatives in biological systems.

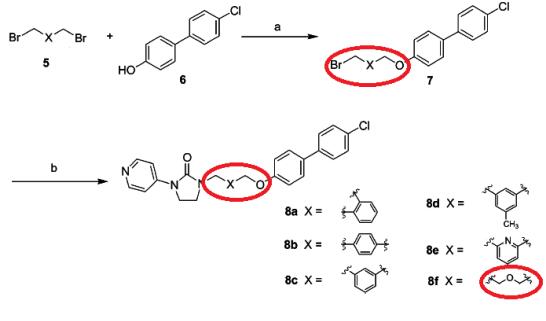
Chang et al. reported [2] its application on the synthesis of 1-[5-(4-Arylphenoxy)alkyl]-3-pyridin-4-ylimidazolidin-2-one derivatives. Compounds 8a-f were synthesized by combining several kinds of commercially available dibromo compounds 5 and 4’-chloro-4-hydroxybiphenyl as starting materials with K2CO3 and KI in N-methylpyrolidinoneat 60 °C to give monosubstituted 7 followed by Nalkylation. It was found that increasing the branched chain to propyl resulted in a progressive decrease in activity, while inserting different heteroatoms entirely rendered the compound only weakly active. The introduction of a bulky group (cyclohexyl, phenyl, or benzyl) led to loss of activity against EV71.
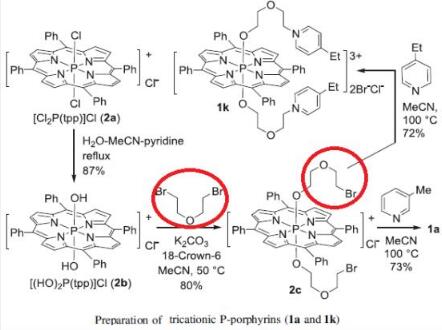
Matsumoto et al. reported [3] its application on the synthesis ofOptimal axial alkylpyridinium-bonded tricationic P-porphyrin. These watersoluble porphyrins were applied to sensitize the inactivation of Escherichia coli under visible-light irradiation, since there are only few 1O2 sensitizers that can efficiently inactivate E. coli at low concentrations. Apy-bonded tricationic P-porphyrins could photoinactivate E. coli. The [P] value for E. coli was optimized at bis[5-(3-ethyl-1-pyridinio)-3-oxapentyloxo]tetraphenylporphyrinatophosphorus dibromide, chloride. Polycationic porphyrins have strong binding affinities to DNA and proteins. Alkyl chains might result in moderate hydrophobicity to take advantage of passing through cell wall. It is important to provide the porphyrins with both polycationic character and hydrophobicity for an efficient PDI of E. coli.
References
1.Gierszewski M. et al. Porphyrazines with peripheral isophthaloxyalkylsulfanyl substituents and their optical properties[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2015, 307–308:54–67
2.Chang CS et al. Design, Synthesis, and Antipicornavirus Activity of 1-[5-(4-Arylphenoxy)alkyl]-3-pyridin-4-ylimidazolidin-2-one Derivatives[J]. J. Med. Chem. 2005, 48, 3522-3535
Matsumoto J, Yasuda M. Optimal axial alkylpyridinium-bonded tricationic P-porphyrin in photodynamic inactivation of Escherichia coli Medicinal Chemistry Research, 2018, 27:1478–1484.
);
You may like
Is 1,4-benzoquinone a toxicity compound?
May 11, 2024
The Synthesis method and Toxicity of 18-Crown-6
May 10, 2024
Lastest Price from 2,2'-Dibromodiethyl ether manufacturers
2,2'-Dibromodiethyl ether

US $0.00/KG2023-07-28
- CAS:
- 5414-19-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month
2,2"-Dibromodiethyl ether

US $15.00-10.00/KG2021-07-13
- CAS:
- 5414-19-7
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton


