Uses and Side effects of Pexidartinib
Daiichi Sankyo’s pexidartinib has become the first FDA-approved therapy for tenosynovial giant cell tumour (TGCT), a rare form of tumour affecting joints and tendons.
TGCT – which is also known as pigmented villonodular synovitis (PVNS) or giant cell tumour of the tendon sheath (GCT-TS) – until now had no approved drug therapies, with patients relying on surgery to remove as much of the tumour as possible.
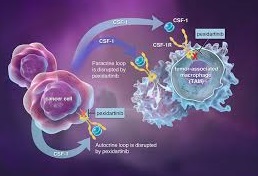
Many tumours recur over time even with surgery, however, and in some cases patients are not eligible for surgical intervention.
In that case they have to live with the sometimes severe symptoms associated with TGCT, including pain, stiffness and restricted movement, which have a significant impact on quality of life and lead to severe disability, according to the FDA. The agency awarded Daiichi Sankyo’s drug breakthrough, priority review and orphan drug status during its development.
Uses
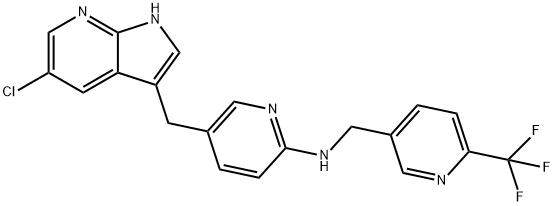
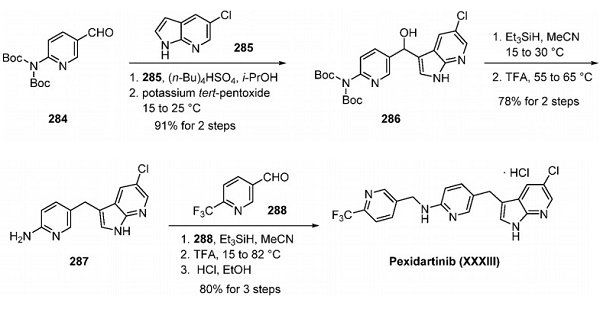
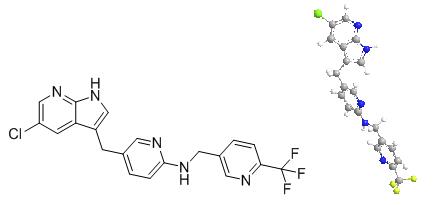
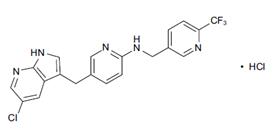
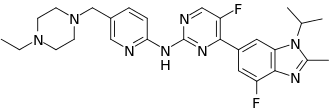
Pexidartinib – which will be marketed under the Turalio trade name in the US – is an oral drug that inhibits CSF1R, KIT and FLT3, three molecular targets that can be used to unlock immune responses to tumour cells.
Its approval is based on a phase III study – ENLIVEN – which was carried out in 120 people with TGCT and found a 38% overall response rate (ORR) with pexidartinib – including 15% complete responses and 23% partial responses – with a zero ORR in a matched placebo group.
Side effects
Common side effects of pexidartinib were increased lactate dehydrogenase, increased aspartate aminotransferase, hair color changes, increased alanine aminotransferase, and increased cholesterol. Additional side effects included neutropenia, increased alkaline phosphatase, decreased lymphocytes, eye edema, decreased hemoglobin, rash, dysgeusia, and decreased phosphate.
The prescribing information includes a Boxed Warning advising health care professionals and patients about the risk of serious and potentially fatal liver injury. Health care professionals should monitor liver tests prior to initiating pexidartinib and weekly for the first 8 weeks, then every 2 weeks for the next month, and, thereafter, every 3 months. In patients experiencing liver injury, the pexidartinib dose should be reduced or discontinued based on severity of the injury. Pexidartinib is available only through a Risk Evaluation and Mitigation Strategy Program.
The recommended pexidartinib dose is 400 mg (2 capsules) orally twice daily on an empty stomach.
);You may like
Related articles And Qustion
See also
Lastest Price from PLX3397 (Pexidartinib) manufacturers
US $1.10/g2021-08-18
- CAS:
- 1029044-16-3
- Min. Order:
- 1g
- Purity:
- 99.9%
- Supply Ability:
- 100 Tons min

US $15.00-10.00/KG2021-07-13
- CAS:
- 1029044-16-3
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton