Unveiling L-Mandelic Acid: A Comprehensive Exploration of its Properties, Synthesis, and Applications
Introduction
L-mandelic acid, also known as levo-mandelic acid, has garnered significant attention within the chemical community for its multifaceted applications and intriguing chemical properties. As a chiral molecule derived from the extraction of amygdalin, L-Mandelic acid serves as a crucial compound in various sectors, including pharmaceuticals, cosmetics, and material science. This article aims to provide an in-depth review of L-Mandelic acid, discussing its synthesis, primary components, uses, and storage methods.

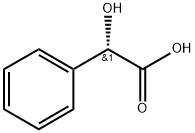
Figure 1 Characteristics of L-Mandelic acid
Synthesis of L-Mandelic Acid
The synthesis of L-mandelic acid typically involves the resolution of racemic mandelic acid or through enzymatic methods that favor the production of the L-enantiomer. One common approach is the enzymatic resolution using lipases, which selectively esterify or hydrolyze one enantiomer over the other. Another method involves the asymmetric synthesis from benzaldehyde using cyanide ions and a chiral catalyst, such as a cinchona alkaloid, which facilitates the formation of L-Mandelic acid directly. These methods ensure the production of L-Mandelic acid with high enantiomeric purity, crucial for its effectiveness in sensitive applications, especially in pharmaceutical formulations where purity is paramount.
Main Components
L-Mandelic acid is a white, crystalline compound that belongs to the alpha-hydroxy acid (AHA) family. Its molecular structure is characterized by a benzene ring substituted with a hydroxyl group and a carboxylic group on adjacent carbon atoms, which is key to its chemical behavior and biological activity. The presence of the chiral center at the alpha position significantly influences its interactions at biological sites, making its pure enantiomeric form especially valuable. This chiral center allows for selective interaction with biological molecules, enhancing its efficacy in various therapeutic and cosmetic applications. Additionally, L-Mandelic acid exhibits higher solubility in water and organic solvents compared to its racemic counterpart, which is beneficial for its incorporation into a wide range of formulations, enhancing its utility in medical and beauty products alike.
Uses of L-Mandelic Acid
L-mandelic acid's applications are vast and varied. In the pharmaceutical industry, it is used as an antibacterial agent and a precursor for the synthesis of various drugs. Its antibacterial properties make it effective in treating urinary tract infections and as a component in topical formulations for acne treatment. Beyond its antibacterial uses, it also shows promise in antifungal treatments and as a potential anti-cancer agent, providing a broad spectrum of therapeutic benefits. In the cosmetic industry, L-mandelic acid is prized for its exfoliating properties, helping in skin rejuvenation and improving skin texture. It accelerates cell turnover, reduces hyperpigmentation, and enhances collagen production, contributing to a more youthful and vibrant skin appearance. Moreover, its ability to stabilize other compounds extends its use to the manufacturing of perfumes and other beauty products, where it acts as a fixative to enhance the longevity and stability of aromatic compounds. This versatility makes L-mandelic acid a valuable ingredient across multiple domains.
Storage Methods
Storing L-Mandelic acid properly is essential to maintain its stability and efficacy. It should be stored in a cool, dry place away from direct sunlight and moisture. The storage containers must be tightly sealed to prevent contamination and degradation of the acid. Under optimal storage conditions, L-Mandelic acid can maintain its effectiveness for a considerable period, making its handling a critical aspect of its application.
Conclusion
L-Mandelic acid continues to play a pivotal role in scientific and industrial applications due to its versatile properties and benefits. The ongoing research and development in its synthesis methods and applications promise to expand its utility even further. For professionals in the chemical field, understanding the nuances of L-mandelic acid is essential for leveraging its full potential in various applications. As we continue to explore this fascinating compound, its contributions to science and industry are bound to increase, marking an exciting path ahead in the realm of chemical research.
References
[1]Pham X H, Kim J M, Chang S M, et al. Enantioseparation of D/L-mandelic acid with L-phenylalanine in diastereomeric crystallization[J]. Journal of Molecular Catalysis B: Enzymatic, 2009, 60(1-2): 87-92.
[2]Xu L, Yang Y, Wang Y, et al. Chiral salen Mn (III) complex-based enantioselective potentiometric sensor for l-mandelic acid[J]. Analytica chimica acta, 2009, 653(2): 217-221.
);You may like
See also
Lastest Price from (S)-(+)-Mandelic acid manufacturers
US $0.00-0.00/kg2024-05-30
- CAS:
- 17199-29-0
- Min. Order:
- 0.10000000149011612kg
- Purity:
- 99
- Supply Ability:
- 20tons

US $0.00-0.00/mg2024-05-14
- CAS:
- 17199-29-0
- Min. Order:
- 10mg
- Purity:
- 0.98
- Supply Ability:
- 10g