Triethylamine Trihydrofluoride: A Versatile Agent in Organic Synthesis
As a fluorination agent, triethylamine trihydrofluoride has incomparable advantages over HF; it is a nearly neutral substance and non-corrosive to borosilicate glass containers; it can be used for substitution reactions of carbohydrate derivatives and aromatic derivatives, and can also be used for addition reactions and desiliconization reactions of olefins.
Synthesis
The synthesis of Triethylamine trihydrofluoride involves a straightforward and cost-effective process. Initially, an organic solvent is added to the first reactor, followed by the addition of HF liquid and triethylamine in a molar ratio ranging from 0.96-1.06:1. The reaction takes place at temperatures between -40°C to 15°C for a duration of 20-720 minutes.
Applications
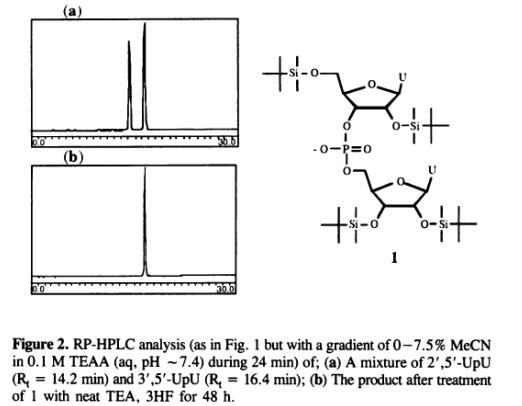
Neat triethylamine trihydrofluoride was reported to be a more efficient desilylating agent than TBAF and successful in deprotecting a synthetic t-RNA (3). When we used TEA, 3HF reliability. The use of this reagent also offers a simpler workup procedure and faster deprotection. 1
As an important organic intermediate, Triethylamine Trihydrofluoride is used in the agrochemical, pharmaceutical and dyestuff fields, amongst others.
Toxicity
Causes burns; Inhalation may cause corrosive injuries to upper respiratory tract and lungs; Highly toxic by ingestion, inhalation, and skin absorption.
References
[1] Gasparutto,4D., Livache,T., Bazin,H., Duplaa,A.-M., Guy,A., Khorlin,A., Molko,D., Roget,A. and Teoule,R. (1992) Nucleic Acids Res. 20,5159-5166.
Related articles And Qustion
See also
Lastest Price from Triethylamine trihydrofluoride manufacturers

US $1.00/KG2025-08-29
- CAS:
- 73602-61-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20T

US $0.00/kg2025-04-18
- CAS:
- 73602-61-6
- Min. Order:
- 1kg
- Purity:
- 97%
- Supply Ability:
- 1000