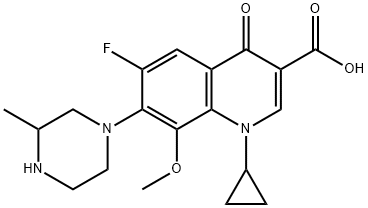
Toxicity of Gatifloxacin
Gatifloxacin is an 8-methoxy fluoroquinolone and is marketed by Bristol Myers Squibb as Tequint. Three formulations are available: oral, ophthalmic, and intravenous. Like other members of this class, gatifloxacin has activity against many Gram-positive and Gramnegative bacteria and intracellular respiratory pathogens. In particular, it has activity against penicillin-susceptible and -resistant Streptococcus pneumoniae, methicillin-susceptible Staphylococcus aureus (MSSA), and some anaerobes. Unlike ciprofloxacin, it lacks significant activity against Pseudomonas aeruginosa.
Mechanism of action
The quinolone class of antibacterials act by inhibition of bacterial topoisomerases. Gatifloxacin inhibits both DNA gyrase (topoisomerase II) and topoisomerase IV. During DNA replication DNA gyrase (a tetramer composed of four subunits, two GyrA and two GyrB) catalyzes ATP-dependent DNA supercoiling. In the contact region of the tetramer, double-stranded DNA is cleaved (the G-segment), while a second region of DNA (the T-segment) is captured and passed through the cleaved DNA gate using ATP as a source of energy. The G-segment is then rejoined and the T-segment exits the tetramer. The DNA gyrase can remain attached to the G-segment with repeated capture of T-segments after each round of ATP hydrolysis.
Like DNA gyrase, topoisomerase IV is a tetramer consisting of four subunits, two each of ParC and ParE, that can remove supercoils. Unlike DNA gyrase, however, topoisomerase IV can also remove precatenanes that are formed behind the replication fork. Fluoroquinolones act by the formation of drug–enzyme–DNA complexes that trap the gyrase and topoisomerase IV on the DNA, resulting in inhibition of DNA synthesis. A second process, fragmentation of the chromosome, results in rapid cell death. Ongoing protein synthesis is necessary for chromosome fragmentation and death after exposure to first-generation quinolones (nalidixic acid). For gatifloxacin, chromosome fragmentation is independent of protein synthesis.
Bioavailability
For oral gatifloxacin, the bioavailability is 96%. In children, the bioavailability of the suspension was similar to the tablet formulation. The degree of protein binding is 20%. The intake of food has no impact on pharmacokinetic parameters such as Cmax, Tmax, t1/2 or volume of distribution. The AUC0–N was only slightly decreased, indicating that gatifloxacin can be administered with or without food. Oral calcium carbonate supplements had no significant effect on the bioavailability or pharmacokinetics of gatifloxacin. Similar results are obtained for ferrous sulfate if given either 2 h before or after oral gatifloxacin. Concomitant administration of mineralcontaining multivitamin preparations may, however, result in treatment failure. The antacid preparation aluminium magnesium hydroxide significantly reduces absorption if given concomitantly or 2 h before gatifloxacin – most likely due to chelation of the antibiotic by polyvalent cations.
Toxicity
Neurotoxicity Fluoroquinolones, particularly older agents, have been reported to possess convulsant activity that may be potentiated by concomitant use of anti-inflammatory drugs. Possible mechanisms include displacement of the neurotransmitter g-aminobutyric acid (GABA), competition for the GABA receptor, or interaction with glutamate receptors, resulting in CNS stimulation.
In a mouse model, a number of fluoroquinolones, including gatifloxacin, have been shown to induce convulsions in a dose-dependent manner. The influence of various anti-inflammatory agents was variable, and for gatifloxacin only weak enhancement occurred with biphenylacetic acid. There has been a limited number of case reports of seizures associated with gatifloxacin use. Other central nervous system side-effects reported for gatifloxacin include psychosis and ataxia.
);You may like
Lastest Price from Gatifloxacin manufacturers
US $0.00/kg2024-04-29
- CAS:
- 112811-59-3
- Min. Order:
- 25kg
- Purity:
- 99.0%
- Supply Ability:
- 10tons

US $0.00/KG2024-03-16
- CAS:
- 112811-59-3
- Min. Order:
- 100g
- Purity:
- 98%+
- Supply Ability:
- 100kg