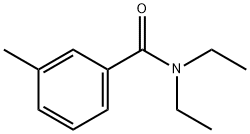
Toxicity of DEET
DEET was first developed and patented by the US Army in 1946. It was approved for general public use by the US Environmental Protection Agency (EPA) in 1957 and was reregistered in 1998. It has been estimated that more than 1.8 million kg (~4 million pounds) of DEET are used in the United States every year in more than 225 registered products. DEET is often sold and used in lotions or sprays with concentrations up to 100%. However, the Center for Disease Control recommends only 30–50% DEET to reduce the incidence of vector-borne disease transmission. Registered products must contain at least 95% of the meta-isomer, but small amounts of the more toxic ortho-isomer and the less toxic para-isomer are permitted.

Use
DEET is used as an insect repellent.
Environmental fate
Little information is available on the environmental fate of DEET. DEET is stable to hydrolysis at environmental pH levels. The initial belief was that DEET was not likely to enter aquatic ecosystems because it was first registered for indoor use. It has been shown in several studies, however, that DEET is found in many waterways in the United States and around the world,such as groundwater, open water, sewage (influent and effluent), surface water, and septic waste in concentrations ranging from 30 ng l-1 to 13 μg l-1. A major source of introduction to aquatic environments is via sewage following washing and excretion by humans.
The potential for DEET to be transported through soil is unknown. Although, some studies have shown that purification of water containing low concentrations of DEET using a combination of sand filtration, activated sludge treatment, and ozonation has a removal efficiency less than 69% (ozonation being the most efficient step). Sand filtration alone was inefficient, notably due to DEET’s hydrophilic nature (Kow < 3). This evidence suggests that DEET may not be retained in soil and other organic matter, but travels with groundwater into larger bodies of water, and more extreme measures than this must be taken to remove DEET from natural and domestic waters. On the other hand, one study noted that DEET ‘has an estimated Koc value of 536, indicating potential for sorption to suspended solids and sediment.’
Mechanism of Toxicity
Historically, it was thought that DEET worked via blocking of insect olfactory receptors and that DEET masked the target to the insect senses so the insect would not detect a food source. Instead, however, recent evidence indicates that the odor of DEET is what acts as the true repellent. A specific type of an olfactory receptor neuron in the antennal sensilla of mosquitoes was identified, and this neuron is activated by DEET. This activity is responsible for the properties that give DEET its repellent ability.
DEET is also toxic to the central nervous system(CNS). DEET
acts as an inhibitor to the enzyme acetylcholinesterase which is
required for the proper functioning of the human nervous
system, other vertebrates, and insects. The enzyme acetylcholinesterase
hydrolyzes acetylcholine, which is important to
muscle control. When this process is inhibited, acetylcholine
builds up in the synaptic cleft and causes neuromuscular paralysis
and death by asphyxiation.
You may like
Lastest Price from N,N-Diethyl-m-toluamide manufacturers

US $0.00/G2024-05-02
- CAS:
- 134-62-3
- Min. Order:
- 1G
- Purity:
- 99%
- Supply Ability:
- 20

US $0.00/KG2024-04-30
- CAS:
- 134-62-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 1000