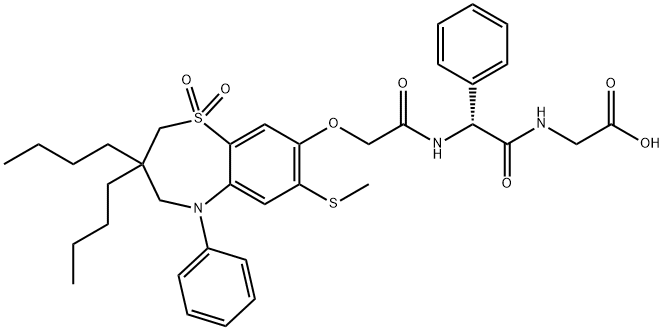
The synthetic route of Elobixibat
Description
Elobixibat (formerly A3309) is an IBAT inhibitor that modulates the enterohepatic circulation of BAs. Elobixibat is a pure enantiomer of a synthetically modified 1,5- 1,5-benzothiazepine based on a seven-membered heterocyclic ring attached to a benzene ring (chemical formula C36H45N3O7S2)[1]. As a highly potent, first-in-class inhibitor of ileal bile acid transport, it is approved for the treatment of chronic idiopathic constipation, a disorder that affects approximately 14% of adults. Elobixibat was developed by EA Pharma (a subsidiary of Eisai Co.) and Mochida and was approved by the Japanese PMDA in 2018. The drug interrupts the enterohepatic circulation of bile while increasing the delivery of bile acids to the colon. These circumstances improve colonic motility and mucosal fluid secretion, resulting in an overall reduction of completely spontaneous bowel movements. Interestingly, multiple biochemical pathways are reported to impact this disease state (e.g., 5-HT4, guanylate cyclase C receptor), necessitating additional studies before the drug’s approval in the United States.
The expected half-life (T1/2) in humans is <4 hours. After oral administration, there is minimal systemic exposure, and the systemically available drug is highly protein-bound (>99.5%). After radiolabeling elobixibat and oral ingestion, there was limited tissue distribution, and it was found only in the gastrointestinal tract at 24 hours.
Synthetic route
General Route to Elobixibat Sulfone (an early-stage intermediate)
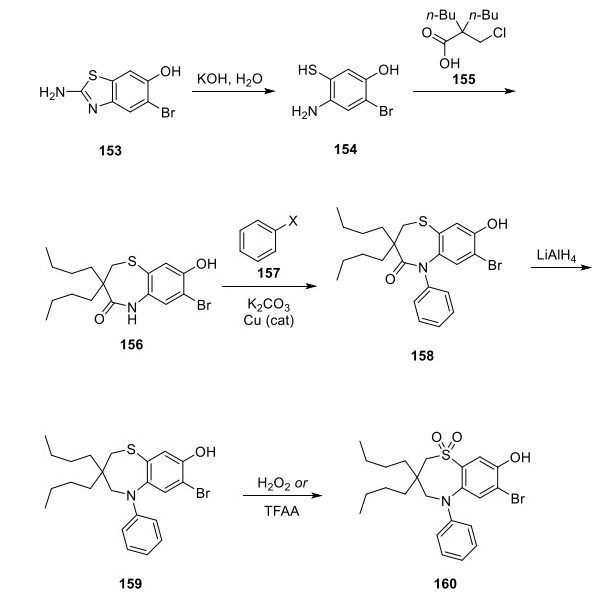
Aminobenzothiazole 153 was hydrolyzed using potassium hydroxide to give mercaptophenol 154, which then underwent a tandem alkylation/lactam formation with α,α-disubstituted- β-halopropanoic acid 155 to form benzothiazepine 156. Copper-catalyzed installation of the N-phenyl group with an unspecified halobenzene (halogen represented by “X” within structure 157) preceded the carbonyl group reduction, resulting in amine 159. This amine was oxidized to sulfone 160 using either hydrogen peroxide or trifluoroacetic acid (TFAA)[2].
Synthesis of Elobixibat Hydrate

A milligram-to-gram-scale conversion of phenol 160 to elobixibat is described in the patent literature. This takes place via sequential aminoester installations, as depicted above. Phenol 160 was alkylated using ethyl bromoacetate (161) to provide ester 162, which was saponified with sodium hydroxide to yield acid 163. Aromatic substitution involving sodium methanethiol prior to amide coupling with (R)-2- phenylalanine methyl ester hydrochloride mediated by 2-(1Hbenzotriazole-1-yl)-1,1,3,3-tetramethylammonium tetrafluoroborate (TBTU) delivered aminoester 165 in high yield. Saponification of the ester gave acid 166, followed by coupling with tert-butyl glycinate with compound 166 and subsequent TFA-mediated tert-butyl cleavage to furnish elobixibat hydrate in 85% yield.
References
[1] Banny S Wong, Michael Camilleri. “Elobixibat for the treatment of constipation.” Expert opinion on investigational drugs 22 2 (2013): 277–84.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
);You may like
See also
Lastest Price from Elobixibat manufacturers

US $12000.00/g2023-11-01
- CAS:
- 439087-18-0
- Min. Order:
- 1g
- Purity:
- 99.0%min, single impurity:0.1%min
- Supply Ability:
- 50kg