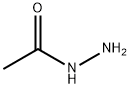
The reactivity of Acethydrazide
Acetohydrazide is a carbohydrazide that is hydrazine in which one of the hydrogens is replaced by an acetyl group and it is a tautomer of an acetohydrazonic acid[1]. Acetohydrazide is an important organic intermediate which is mainly used for the synthesis of compounds with nitrogen heterocycles and acyl azides in the pharmaceutical industry[2].
Why do Acetohydrazide contains good reactivity and acts as active in synthetic and pharmaceutical chemistry? It is necessary to understand the structure of the hydrazine -NH-NH2 moiety in acid hydrazides.
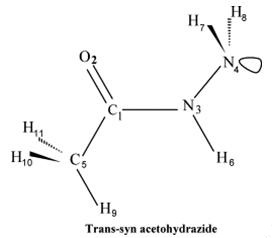
Scheme 1 Trans-syn structure of acetohydrazide
Researchers[3] studied the structural character of acetohydrazide by scanning the potential energy of structure with Gaussian software. The bonds have stored chemical potential energy which is released when they are broken, and the substance undergoes a chemical reaction. Atoms in a chemical bond are more stable and have lower potential energy than. After energy scanning, the C-N rotational barrier in the molecule was calculated to be about 26 kcal/mol that suggested the planar sp2 nature of the nitrogen atom of the central NH moiety. The N atom of the terminal NH2 group was predicted to highly prefer sp3 structure with an inversion barrier of about 7~8 kcal/mol. The molecule was predicted to have a trans-syn (N-H bond is trans with respect to C=O bond and NH2 moiety is syn to C-N bond) conformation as the lowest energy structure.
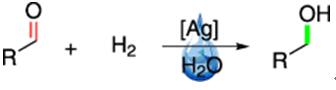
The reactivity of acetohydrazide can be extended to acetohydrazide derives and hydrazides. There is a classical reaction shows the reactivity of acetohydrazide/ hydrazine clearly. The Schiff-base reaction (scheme 2) refers to the reaction between the class of substances containing carbonyls (aldehydes and some ketones) with amino groups (primary amine, hydroxylamine, hydrazine, etc.).
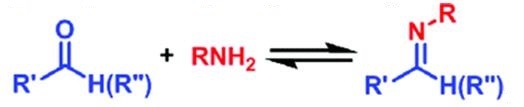
Scheme 2 Schiff-base reaction
In the reaction, simply mixing and stirring the two reactants can result in an easy reaction and produce the target product in very high yields. Simple primary amines, hydroxy-substituted hydroxylamines, and hydrazides are three commonly used amino-containing reactants. The imine formed between a primary amine and an aldehyde group is less stable. However, with the other amines, the imine C=N double bonds formed are more stable. These C=N bonds only break when the pH value falls below 3. In systems where high stability is required, hydroxy-substituted hydroxylamines and hydrazides are the amines of choice [4].
Reference
[1] https://fabricheminc.com/specialty-fine-chemicals/acetic-hydrazide/
[2] M. Yadav etc., Synthesis and application of new acetohydrazide derivatives as a corrosion inhibition of mild steel in acidic medium: insight from electrochemical and theoretical studies, Journal of Molecular Liquid, Volume 208, August 2015, p 322-332.
[3] Hassan M.Badawi, Vibrational spectra and analysis of acetohydrazide CH3–CO–NH–NH2, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Volume 67, Issues 3–4, July 2007, Pages 592-597
[4] Yan Xin and Jinying Yuan, Schiff's base as a stimuli-responsive linker in polymer chemistry, Polym. Chem., 2012, 3, 3045-3055.
You may like
See also
Lastest Price from Acethydrazide manufacturers

US $30.00/kg2024-05-21
- CAS:
- 1068-57-1
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000kg
US $50.00-1.00/kg2024-03-25
- CAS:
- 1068-57-1
- Min. Order:
- 1kg
- Purity:
- 99
- Supply Ability:
- g-kg-tons,free sample is available