The Pharmacokinetics and toxicity of Mesalazine
Description
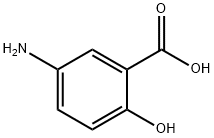
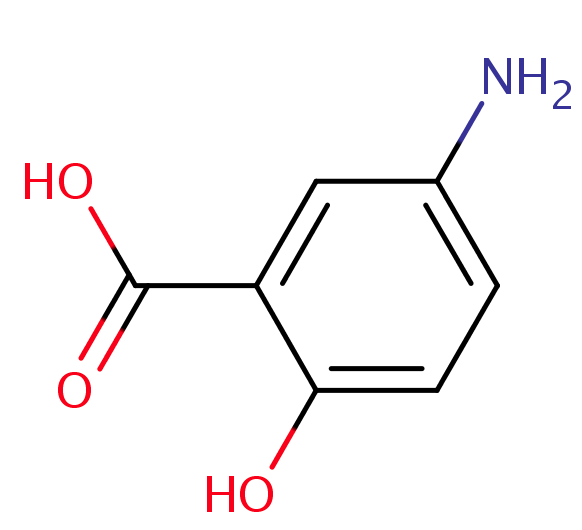
Mesalazine, also known as mesalamine or 5-aminosalicylic acid (5-ASA), is a medication commonly used for the treatment of inflammatory bowel disease. It was approved for medical use in the United States in 1987. Over the years, there has been significant research on the toxicity and pharmacokinetics of this drug. The absorbed part of mesalazine is almost completely acetylated in the gut wall and in the liver to acetyl-5- aminosalicylic acid. The acetylated metabolite is excreted mainly in urine by tubular secretion, with traces of the parent compound.

Pharmacokinetics
In a study involving volunteers who were given a single oral dose of prolonged-release mesalazine 250mg, the median lag time (tlag) to the first detectable plasma concentration of mesalazine was 45 minutes, with a range of 15 to 150 minutes. The maximum plasma concentration (Cmax) of mesalazine was recorded as 0.6 μmol/L, with a range of 0.4 to 1.4 μmol/L, which occurred 240 minutes after administration. Similarly, acetyl mesalazine had a tlag of 22 minutes (15 to 45 minutes), Cmax of 2.9 μmol/L (1.6 to 3.4 μmol/L), and time to reach maximum concentration (tmax) of 105 minutes (60 to 300 minutes)[1].
In another study involving healthy volunteers given a single oral dose of prolonged-release mesalazine 1g, the drug exhibited continuous release throughout the gastrointestinal tract. Plasma concentrations peaked at 0.53 mg/L 4 hours after administration, declined rapidly to 0.03 mg/L at 12 hours, then remained fairly constant over the next 24 hours before resuming the final decline, becoming undetectable 60 hours after administration. The area under the plasma concentration-time curve (AUC) for mesalazine was 4.37-mg/L • h. Little is known about the distribution of prolonged-release mesalazine. In 9 pregnant women with IBD who were receiving prolonged-release mesalazine 0.5 to 3 g/day, low concentrations (approximate values from graph) of mesalazine and acetyl mesalazine were measured in maternal (≤0.5 and ≤7.5 μmol/L) and fetal plasma (≤0.25 and ≤18 μmol/L). In 2 patients, low concentrations of mesalazine were detected in breast milk. Mean acetyl mesalazine concentrations in breast milk were 4.4 to 47.5 μmol/L[2-3].
Mesalazine is primarily metabolized by acetylation in the gut wall and the liver, forming the therapeutically inert metabolite acetyl mesalazine. Both the parent compound and the metabolite are excreted in the urine.[4] After a single oral administration of prolonged-release mesalazine 0.25g in 6 volunteers, the apparent mean elimination half-life of acetyl mesalazine was 802 minutes (range 608 to 993). Determination of the terminal half-life of mesalazine was not possible because of low plasma concentrations. After oral administration of prolonged-release mesalazine 1.5 to 4 g/day to volunteers, excretion of unchanged mesalazine accounted for 8 to 12% of the daily dose. Total urinary excretion of mesalazine plus acetyl mesalazine was 29 to 53%[5]. In volunteers, renal clearance of acetyl mesalazine was 12 L/h (201 ml/min) at steady state. In a 7-day study of 15 patients with ulcerative colitis, daily urinary excretion of mesalazine and acetyl mesalazine was higher with prolonged-release mesalazine (1.5 g/day) and pH-dependent delayed-release mesalazine (Asacol ®, 1.2 g/day) than with olsalazine (1 g/day).
Side effects and toxicity
In an 8-week randomized trial, 314 patients with ulcerative colitis were divided into four groups: one group received prolonged-release mesalazine at a dosage of 1g/day, another at a dosage of 2g/day, another at a dosage of 4g/day, and the last group received a placebo. The trial found that 16% of patients in the active drug groups experienced treatment-related adverse events, compared to 22% in the placebo group. The study did not find a dose-response relationship. Additionally, 5%, 9%, and 7% of patients in the 1g/day, 2g/day, and 4g/day groups, respectively, discontinued therapy due to either treatment-related or unrelated events. In contrast, 12% of placebo-treated patients discontinued therapy. Diarrhea, abdominal pain, fever, and melaena were the most common treatment-limiting adverse events observed[6].
In another study that lasted 16 weeks, the most common adverse events related to prolonged-release mesalazine treatment were nausea and/or vomiting (7.4% compared to 3.7% in the placebo group), headache (5.2% compared to 3.7%), and abdominal pain (4.3% vs 5.0%)[7].
In a 12-month study involving 205 patients with ulcerative colitis, adverse events necessitating withdrawal occurred in 14% and 33% (2% and 6% considered to be treatment-related) of patients receiving prolonged-release mesalazine 4 g/day and placebo, respectively. Treatment-related adverse events (most commonly nausea 2.9%, abdominal pain 1.9%, and dyspepsia 1.9%) were experienced in 6.8% of patients receiving prolonged-release mesalazine. In contrast, 11.8% of patients in the placebo group experienced adverse events related to therapy[8]. In a non-comparative study of 467 patients with Crohn’s disease who received prolonged-release mesalazine at dosages up to 4 g/day for a median of 14 months, 12%of patients discontinued because of treatment-related adverse events, of which the most commonly reported were diarrhea (4.3%), abdominal pain (3.6%) and dyspepsia (3.1%)[9].
References
[1] Bondesen S, Hegnhoj J, Larsen F, et al. Pharmacokinetics of 5-aminosalicylic acid in man following administration of intravenous bolus and Per Os slow-release formulation. Dig Dis Sci 1991; 36: 1735-40.
[2] Staerk-Laursen L, Stokholm M, Bukhave K, et al. Disposition of 5-aminosalicylic acid by olsalazine and three mesalazine preparations in patients with ulcerative colitis: comparison of intraluminal colonic concentrations, serum values, and urinary excretion. Gut 1990; 31: 1271-6.
[3] Christensen LA, Rasmussen SN, Hansen SH. Disposition of 5-aminosalicylic acid and N-acetyl-5-aminosalicylic acid in fetal and maternal body fluids during treatment with different 5-aminosalicylic acid preparations. Acta Obstet Gynecol Scand 1994; 74: 399-402.
[4] Lauritsen K, Laursen LS, Rask-Madsen J. Clinical pharmacokinetics of drugs used in the treatment of gastrointestinal diseases (Part II). Clin Pharmacokinet 1990; 19: 94-125.
[5] Rasmussen SN, Bondesen S, Hvidberg EF, et al. 5-Aminosalicylic acid in a slow-release prepararation: bioavailability, plasma level, and excretion in humans. Gastroenterolo 1982; 83: 1062-70.
[6] Hanauer S, Schwartz J, RobinsonM, et al.Mesalamine capsules for the treatment of active ulcerative colitis: results of a controlled trial. Am J Gastroenterol 1993; 88: 1188-97.
[7] Miner P, Hanauer S, Robinson M, et al. Safety and efficacy of controlled-release mesalamine for maintenance of remission in ulcerative colitis. Dig Dis Sci 1995; 40: 296-304.
[8] Singleton JW, Hanauer SB, Gitnick GL, et al. Mesalamine capsules for the treatment of active Crohn’s disease: results of a 16-week trial. Pentasa Crohn’s Disease Study Group[see comments]. Gastroenterology 1993; 104: 1293-301.
[9] Hanauer SB, Krawitt EL, Robinson M, et al. Long-term management of Crohn’s disease with mesalamine capsules (Pentasa ®). Am J Gastroenterol 1993; 88: 1343-51.
Related articles And Qustion
See also
Lastest Price from 5-Aminosalicylic acid manufacturers

US $0.00/kg2025-05-07
- CAS:
- 89-57-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 1000kg

US $0.00-0.00/Kg/Drum2025-04-21
- CAS:
- 89-57-6
- Min. Order:
- 1KG
- Purity:
- 98.5%~101.5%; USP40
- Supply Ability:
- 15tons/month