The introduction of licochalcone C
General description
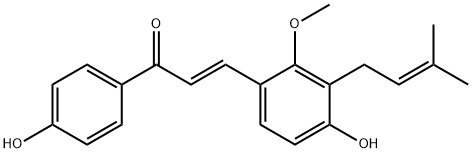
The licochalcone C, with the CAS No: 144506-14-9, is also known as 4,4'-Dihydroxy-2-Methoxy-3-prenylchalcone. This chemical’s molecular formula is C21H22O4 and molecular weight is 338.4. Licochalone C is a retrochalcone isolated from Glycyrrhiza inflata (Figure 1), which shows potent antioxidant properties and inhibition of bacterial growth and cellular respiration. licochalcone C attenuates the lipopolysaccharide and interferon- gamma induced inflammatory response by decreasing the expression and activity of inducible nitric oxide synthase and modulating the antioxidant network activity of superoxide dismutase, catalase, and glutathione peroxidase activity [1].

Figure 1 Figure of Glycyrrhiza inflata.
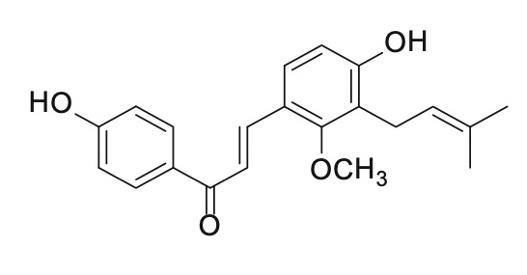
Figure 2 The molecular formula of licochalcone C
Bioactivities
The constituent of G. inflata, licochalcone C, was effective in preventing microsomal lipid peroxidation induced by Fe (III)-ADP/ NADPH and showed potent antioxidative and superoxide scavenging activities [2]. Meanwhile, licochalcone C possess anti-inflammatory activity. It decreases the expression and activity of iNOS and modulates the antioxidant network activity of SOD, catalase, and glutathione peroxidase activity [3]. Licochalcone C significantly inhibited the production of free radicals in LPS-treated Raw 264.7 macrophages model [4]. Licochalcone C blocks cell cycle progression at the G2/M transition, inhibits the expression of uridylyl phosphate adenosine, phosphor- JNK, and phosphor-map kinase kinase 4, reactives the ROS- dependent pathway, and induces cancer cell apoptosis by an endoplasmic reticulum stressor through a caspase-dependent FasL-mediated death receptor pathway [5,6]. It leads to S phase arrest, enhances Bax expression, activates caspase-3, cleaves the poly ADP-ribose polymerase protein, and decreases the expression of cyclin A, CDK1 and CDK2 mRNA, cell division cycle 25 (Cdc25A and Cdc25B) protein, Bcl-2, and surviving [7]. Licochalcone C induced T24 cell a tosis in a concentration- dependent manner. Licochalcone C treatment reduced the levels of the anti-apoptotic mRNAs (Bcl-2, Bcl-w and Bcl-XL) and increased expression of the pro -apoptotic mRNAs (Bax and Bim). The Bcl-2 family inhibitor (ABT-737) reduced apoptosis induced by licochalcone C in T24 cells. The current study demonstrated that licochalcone C may be a potential adjuvant therapeutic agent for bladder cancer [8]. In addition, licochalcone C also showed immunoregulatory activity. Licochalcone C inhibits H2O2, NO, IFN-γ, TNF-α, and IL-17 production, and modulates the immune response on both Th1 and Th17 cells.
Pharmacodynamics
In an in vivo pharmacokinetic study after oral administration of licochalcone C to SD rats (200 mg/kg), Cmax was 3.31 ± 0.30 μg/ml, AUC0-24 h was 21.83 ± 1.44 μg/ml, T1/2 was 7.21 ± 0.37 h, MRT was 10.26 ± 1.01 h [9]. Furthermore, Liu and others found a Cmax of 3.39 ± 0.22 μg/ml, AUC0-24 h of 19.37 ± 0.56 μg/ml, T1/2 value of 7.43 ± 0.51 h, MRT of 11.84 ± 0.67 h, and Tmax of 2 ± 0 h. Meanwhile, licochalcone C is rapidly distributed to all tissues, particularly in the liver, kidney, and spleen, within a short time [5].
Toxicity
It has been reported that large doses or long-term injections of licochalcone C sometimes produce an acquired form of apparent mineralocorticoid excess syndrome, expressed as sodium retention, hypokalemia, and high blood pressure [10]. According to a recent report, the medical records of patients treated with herbal complexes containing licochalcone C from January 1, 2010 to December 31, 2010 were examined. The changes in the levels of creatinine, potassium, and blood urea nitrogen before and after herbal complex intake were recorded, and the prevalence of hypokalemia among these patients were investigated. Three hundred and sixty patients did not show significant changes in the levels of potassium and creatinine (p = 0.815 and 0.289, respectively) and hypokalemia was observed in six patients. However, in five patients, the hypokalemia did not appear to be related to the licochalcone C. This investigation suggested that herbal complexes containing licochalcone C did not significantly influence the potassium levels in routine clinical herbal therapies [11].
References
[1]Na Y, Cha J H, et al. Concise synthesis of licochalcone C and its regioisomer, licochalcone H. Arch. Pharm. Res. (2013) 36:1432–1436.
[2]Haraguchi H, Ishikawa H, Mizutani K, Tamura Y, Kinoshita T. 1998. Antioxidative and superoxide scavenging activities of retrochalchones in Glycyrrhiza inflata. Bioorg Med Chem 6: 339–347.
[3]Wang Z, Cao Y, Paudel S, Yoon G, Cheon SH. Concise synthesis of lico- chalcone C and its regioisomer, licochalcone H. Arch Pharm Res 2013; 36: 1432–1436
[4]Lee H N, Cho H J, Lim D Y, et al. Mechanisms by which licochalcone E exhibits potent anti-inflammatory properties: studies with phorbol ester-treated mouse skin and lipopolysaccharide-stimulated murine macrophages. Int J Mol Sci. 2013,14:10926-10943.
[5]Choi AY, Choi JH, Hwang KY, Jeong YJ, Choe W, Yoon KS, Ha J, Kim SS, Youn JH, Yeo EJ, Kang I. Licochalcone A induces apoptosis through endoplas- mic reticulum stress via a phospholipase Cgamma1-, Ca(2+)-, and reac- tive oxygen species-dependent pathway in HepG2 human hepatocel- lular carcinoma cells. Apoptosis 2014; 19: 682–697
[6]Kim JS, Park MR, Lee SY, Kim DK, Moon SM, Kim CS, Cho SS, Yoon G, Im HJ, You JS, Oh JS, Kim SG. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. On- col Rep 2014; 31: 755–762
[7]YuanX,LiT,XiaoE,ZhaoH,LiY,FuS,GanL,WangZ,ZhengQ,WangZ. Licochalcone B inhibits growth of bladder cancer cells by arresting cell cycle progression and inducing apoptosis. Food Chem Toxicol 2014; 65: 242–251
[8]Wang P, Yuan X, Wang Y, et al. Licochalcone C induces apoptosis via B-cell lymphoma 2 family proteins in T24 cells. Molecular Medicine Reports, 2015, 12: 7623-7628.
[9]Zhu, Z., Liu, J., Yang, Y., Adu-Frimpong, M., Ji, H., Toreniyazov, E., et al. (2021). SMEDDS for Improved Oral Bioavailability and Anti-hyperuricemic Activity of Licochalcone A. J. Microencapsul. 38 (7-8), 459–471.
[10]Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer 2001; 39: 1–11.
[11]Jung W, Kwon S, Im J, Park S, Moon S, Park J, Ko C, Cho K. Influence of herbal complexes containing licorice on potassium levels: a retro- spective study. Evid Based Complement Alternat Med 2014; 2014: 970385.
);You may like
Related articles And Qustion
See also
Lastest Price from LICOCHALCONEC manufacturers

US $0.00/kg2024-04-12
- CAS:
- 144506-14-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton
US $0.00/mg2023-02-24
- CAS:
- 144506-14-9
- Min. Order:
- 10mg
- Purity:
- ≥98%(HPLC)
- Supply Ability:
- 10 g