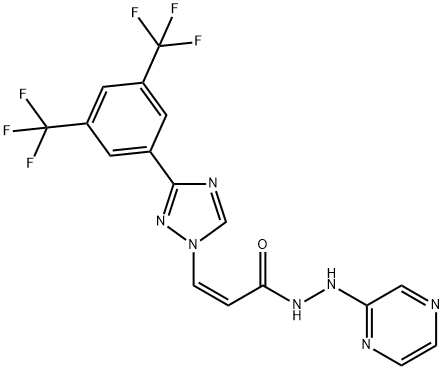
The introduction of KPT-330(Selinexor)
Description
Selinexor (KPT-330) is a first-in-class, oral, small molecule Exportin-1 (XPO1) inhibitor that is being developed by Karyopharm Therapeutics for the treatment of cancer[1]. Selinexor (in combination with dexamethasone) received accelerated approval in the USA in July 2019 for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM). Selinexor is also undergoing clinical development in various haematological and solid cancers.
Biological activity
Selinexor is an anti-cancer drug that inhibits nuclear exportin-1 (XPO1), inhibiting the transport of nuclear proteins from the nucleus to the cytoplasm, leading to the induction of cell-cycle arrest and apoptosis. XPO1 inhibitors had antiviral effects, mainly against respiratory syncytial virus (RSV) and influenza virus. In particular, selinexor inhibits the transport of SARS-CoV-2 nuclear proteins to the cytoplasm, further inhibiting SARS-CoV-2 proliferation. This drug can prevent the development of a cytokine storm in COVID-19 by inhibiting the release of pro-inflammatory cytokines with the augmentation release of anti-inflammatory cytokines. In conclusion, SARS-CoV-2 infection is linked with the activation of XPO1, triggering inflammatory reactions and oxidative stress. Inhibition of XPO1 by Selinexor, a selective inhibitor of nuclear export (SINE), can reduce the proliferation of SARS-CoV-2 and associated inflammatory disorders[2].
Synthesis method
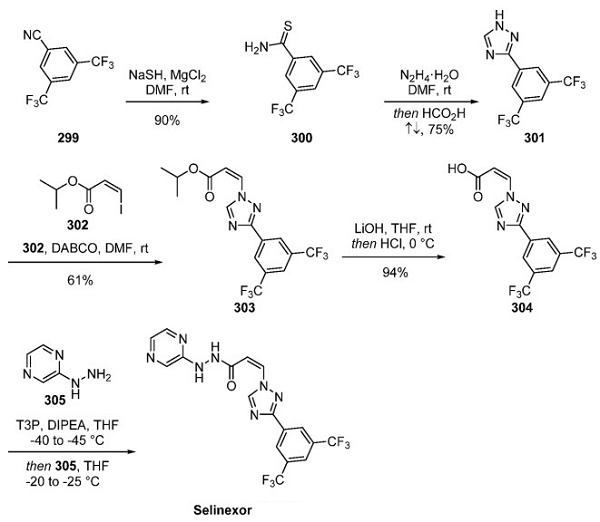
In a synthetic route to selinexor disclosed in an early patent from Karyopharm, many reactions within the sequence were exemplified on a 100 g scale, and this route is depicted above[3]. The route began with the reaction of 3,5- bis(trifluoromethyl)benzonitrile (299) with sodium hydrosulfide and magnesium chloride in DMF to generate thioamide 300, which upon subjection to hydrazine hydrate in DMF underwent a cyclization reaction to furnish 1,2,4-triazole 301. Next, adding vinyl iodide 302 and subsequent elimination gave rise to 303, which was reported to exist as a 9:1 ratio of the desired Z isomer to the E isomer impurity. It is unclear from reports whether purification methods removed the E isomer or if this isopropyl ester is prone to isomerization. Ester 303 was saponified under basic conditions, and after pH adjustment, T3P-mediated coupling with hydrazine 305 generated a selinexor.
References
[1] Syed, Yahiya Y. “Selinexor: First Global Approval.” Drugs 79 1 (2019): 1485–1494.
[2] Gomaa Mostafa-Hedeab. “Selinexor and COVID-19: The Neglected Warden.” Frontiers in Pharmacology 13 (2022): 884228.
[3] Andrew C. Flick. “Synthetic Approaches to the New Drugs Approved during 2019.” Journal of Medicinal Chemistry 64 7 (2021): 3604–3657.
You may like
See also
Lastest Price from Selinexor manufacturers

US $0.00/g2025-01-13
- CAS:
- 1393477-72-9
- Min. Order:
- 1g
- Purity:
- More Than 99%
- Supply Ability:
- 100kg/Month

US $0.00-0.00/g2024-11-01
- CAS:
- 1393477-72-9
- Min. Order:
- 1g
- Purity:
- 98%+
- Supply Ability:
- 100kgs