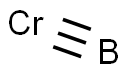The Crystal structure of Chromium boride
Desription
The multicomponent Ni-based alloys composed of Cr, Fe, Mo, W, Si, B, and so on, with high hardness, melting points, acid resistance, and oxidation resistance, have been used as corrosion-, wear-, and heat-resistant materials. In the Cr-B binary phase diagram, CrB, Cr2B, Cr5B3, Cr3B4, CrB2, and CrB4 are the six stable phases, and CrB2 has the highest melting point (2200 • C). Chromium borides usually have superior properties such as high chemical stability, melting point, thermal conductivity, hardness and strength, and relatively low thermal expansion[1]. Cr3B4, CrB2, and CrB have high-temperature strength and stability, so they are especially used as structural materials. Chromium borides are not only used for high-temperature applications but are also used as corrosion-resistant hard coatings and as additives to improve the mechanical, electrical, and corrosion-resistance properties of the composites.
Crystal structure

Figure shows that each Cr atom is surrounded by seven B atoms with three types denoted as B1, B2, and B3. The B1 atoms, which form a tetragonal plane parallel to the a-c plane, are closest to Cr atoms with a distance of 2.19 A˚, representing the strongest bonding between the Cr and B atoms. Cr-B2 and Cr-B3 bonds have longer distances of 2.22 A˚ and 2.29 A˚, respectively, suggesting weaker interactions. On the other hand, the B1-B2 bonds form zigzag boron chains with a bonding distance of 1.78 A˚ in the b-c plane, which is much shorter than that in WB.15,20 This may play an important role in resisting plastic deformation[2].

Nanocatalyst
Chromium boride (CrB) nanocatalyst was developed to power superior Li–S battery chemistry. The CrB catalyst with unique laminated nanostructure was prepared via a well-confined, mild-temperature, and solid-state thermal method. Benefiting from its 2D interstitial-alloy lattice structure, CrB inherits good metallicity with a high conductivity of 357 S m−1. More importantly, the unique dual-sulphophilic character of CrB imposes simultaneous p-d and p-p hybridizations with LiPS, which is distinct from the Lewis acid-base mode in conventional catalyst scenarios and enables strong sulfur immobilization against the shuttle effect and lowered energy barriers for multistep sulfur conversions. As a result, fast, efficient, and durable sulfur redox electrochemistry was realized, instantiated by the excellent cyclability over 2000 cycles, superb rate capability up to 7 C, and decent energy output even under the high loading (sulfur loading = 5.0 mg cm−2), and lean-electrolyte (E/S ratio = 5.5 mL g−1).
References
[1] Hongyang Li. “Dually Sulphophilic Chromium Boride Nanocatalyst Boosting Sulfur Conversion Kinetics Toward High-Performance Lithium–Sulfur Batteries.” Advanced Science 10 32 (2023).
[2] Lei Han. “Hardness, elastic, and electronic properties of chromium monoboride.” Applied Physics Letters 106 1 (2015): 221902.