The Chemical Properties of Propane
Description
Propane (C3H8) is one of the most versatile fuels used in the United States. Propane is used at home for heating water, heating spaces, cooking, drying clothes, and barbecuing. It is also used in recreation for gas grills, outdoor gas lights, mobile homes, RV appliances, generators, and swimming pools[1]. This compound is a staple on 660,000 farms for crop drying and flame cultivation.
Chemical property
Propane is a colorless and odorless flammable gas. It is also an asphyxiant. If inhaled, it will cause dizziness, difficulty breathing, or loss of consciousness. Vapors are an irritant to the eyes and throat. However, vaporizing liquid can also cause frostbite. The main hazard of Propane is its highly flammable nature. Flammable Propane can cause injuries due to burns, thermal radiation, and carbon monoxide poisoning. Since the gas is heavier than air, it may accumulate in low spaces and near the space floor. It can travel far to find the ignition source and flash back. Containers with propane gas can explode in terms of fireballs or vapor cloud explosions. Propane can also explode in confined spaces.
Polar or non-polar?
Each C-H bond in the C3H8 molecule is non-polar due to a slight electronegativity difference between the bonded atoms. Each C-C bond is also non-polar due to no electronegativity difference between them.
The electronegativity of the carbon (C) atom is only slightly more than the hydrogen (H) atom. There is almost equal sharing of the electron cloud in the C-H bond due to this slight electronegativity difference. Thus, each C-H and C-C bond in the C3H8 molecule is non-polar and possesses no dipole moment value.
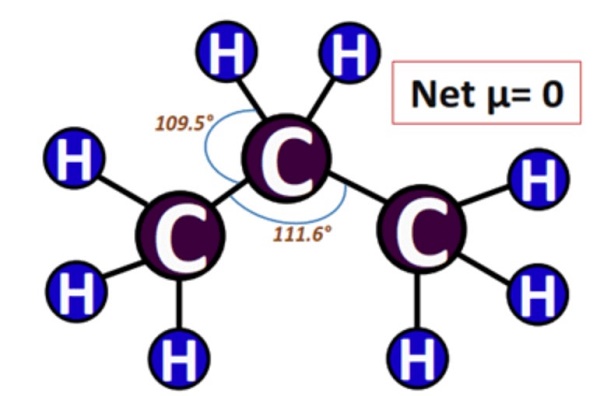
In addition, due to the symmetrical tetrahedral geometry of C3H8, the net dipole moments (µ), if any, will be canceled.
As discussed above, the propane molecule is non-polar. The difference in electronegativity is less than 0.5; in this molecule, it is 0.3. Furthermore, the molecular geometry is symmetrical and possesses no lone pairs. Therefore, the propane molecule is non-polar.
References
[1] Nitin Roy. “Patterns and Trends in Injuries Due to Consumer Propane Incidents.” ACS Chemical Health Safety 27 4 (2020): 251–258.
);