The brief introduction of NADPH
Introduction
Reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases are a group of plasma membrane-associated enzymes, among which the phagocytic leukocyte NADPH oxidase has been most often studied. During phagocytosis or exposure to other activating agonists, the NADPH oxidase of neutrophils and other professional phagocytic cells (including monocytes) catalyzes the production of superoxide anion (O2-) by the one-electron reduction of molecular oxygen. The enzyme complex uses NADPH, provided by the pentose phosphate pathway, as the electron donor[1].
NADPH provides reduction equivalents for GSH regeneration. Two main contributors to the NADPH cytosolic reduction equivalents are the pentose phosphate pathway and a serine carbon metabolism (folate cycle). Other NADPH generators include ME1, IDH1/2, and NNT. NADPH is used as an anabolic pathway for lipid synthesis, cholesterol synthesis, and lengthening of the fatty acid chain (its most important function and not the redox function). The increased demand for NADPH-producing enzymes in cancer cells may reflect an increase in redox support and an increase in anabolic demands.
NADPH-dependent c in cancer
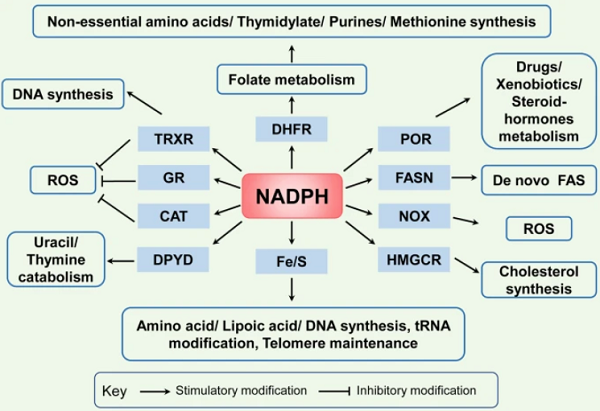
NAD(H) and NADP(H) are cofactors used to transfer and reserve reduction potential. However, closely related structures, NAD(H) and NADP(H), are recognized by unique compartmentalized enzymes and exert different functions. NAD(H) is mainly involved in catabolic reactions, whereas NADP(H) is primarily involved in cellular antioxidative effects and anabolic reactions.
Antioxidative effects
In cancer cells, overcoming oxidative stress is critical for tumor progression. NADPH plays a crucial role in cellular antioxidation systems by providing reducing equivalents to generate reduced forms of antioxidant molecules, which are highly corrected with cancer cell biological behaviors. On the one hand, GSH reductase converts GSSG to GSH using NADPH as an essential cofactor. GSH acts as a cosubstrate for GSH peroxidase (GPX) that reduces hydrogen peroxide (H2O2) and other peroxides to H2O or alcohol to deactivate ROS. On the other hand, TRX reductase (TRXR) utilizes NADPH as an electron donor to maintain the reduced form of TRX, which contributes to scavenging H2O2 and reducing ribonucleotide reductase (RNR) for DNA synthesis. In addition, in some cell types, NADPH binds to the critical H2O2-disposing enzyme, catalase, and reactivates it when it has been inactivated by H2O2[2].
Reductive synthesis
NADPH is also a crucial electron source for several reductive synthesis reactions, including fatty acids, amino acids, nucleotides, and steroid synthesis, to sustain rapid tumor cell growth. Primarily, NADPH provides reducing equivalents for fatty acid synthase (FASN), the main rate-limiting enzyme, to synthesize fatty acids with acetyl-CoA serving as a primer and malonyl-CoA as a two-carbon donor and provides the needed electrons for iron-sulfur (Fe/S) protein assembly that participates in non-essential amino acid biosynthesis and lipoic acid synthesis, tRNA modification, DNA replication and repair, and telomere maintenance. NADPH is also needed for the dihydrofolate reductase (DHFR) enzyme to catalyze the reduction of dihydrofolate to tetrahydrofolate (THF) in folate metabolism, which is required for de novo biosynthesis of thymidylate, purines, methionine, and some amino acids. Besides, NADPH acts as the reducing reagent for 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), the rate-limiting enzyme of the mevalonate pathway, which leads to the synthesis of cholesterol and nonsterol isoprenoids. NADPH also acts as a cosubstrate for dihydropyrimidine dehydrogenase (DPYD), which catalyzes the reduction of uracil and thymine to 5,6-dihydrouracil and 5,6-dihydrothymine, respectively. In addition, the cytochrome P450 reductase (POR) activity also requires NADPH, which has a significant role in the metabolism of drugs, xenobiotics, and steroid hormones.
Free radical generation
In addition, NADPH is also responsible for the generation of free radicals by NADPH oxidases (NOX) as a substrate. NOXs (NOX1–5 and dual oxidases (DUOX) 1 and 2) catalyze the superoxide anions or H2O2 from NADPH and oxygen. NOX-mediated ROS broadly and specifically regulate various redox-sensitive signaling pathways involved in cancer progression via stimulating oncogenes, such as Src and Ras, and inactivating tumor suppressor proteins, such as TP53 and PTEN.
References
[1] E. Bey, M. Cathcart. “Antisense oligodeoxyribonucleotides: a better way to inhibit monocyte superoxide anion production?” Methods in enzymology 353 1 (2002): 421–34.
[2] Huai-Qiang Ju. “NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications.” Signal Transduction and Targeted Therapy (2020): 231.
See also

US $0.00/KG2025-04-15
- CAS:
- 53-57-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg

US $0.00/KG2025-03-21
- CAS:
- 53-57-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month