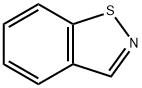
Synthesis of Benzisothiazole
The parent skeleton of benzisothiazole is a bicyclic structure in which the benzene ring is fused with the “d” site of the isothiazole ring and is also known as benzo[d]isothiazole. The compounds containing the benzisothiazole framework are biologically very important because they exhibit herbicidal and antimicrobial properties. These molecules have demonstrated their ability to inhibit the carbonic anhydrase isoform IX (CAIX) and stop cell proliferation under hypoxic conditions. In addition, a benzisothiazole derivative, perospirone, has been developed as serotonin 5-HT2 and dopamine D2 antagonists, which are used for the treatment of schizophrenia in Japan. Various compounds of the benzisoxazole ring in clinical use are listed in the following diagram.
Synthesis
Due to the interesting biological significance of this class of compounds, efforts have been made to develop diverse synthetic methods for the construction of a wide range of benzisothiazole derivatives. The most usual general strategies for the synthesis of these molecules are presented next.
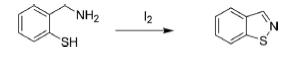
1. From 2-Mercaptobenzylamine
The compound undergoes oxidative cyclization in the presence of molecular iodine to generate benzo[d]isothiazole.
However, the corresponding benzo[c]isothiazoles can be prepared via H2O2 oxidation of 2-aminothiobenzamides.

2. From Sulfenyl Chlorides
2-Acylphenylhypochlorothioite successfully undergoes intramolecular cyclization after reaction with ammonia to produce various benzo[d]isothiazoles.