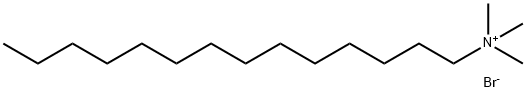
Synthesis and Application of Cetrimide
General description
Cetrimide, also called tetradecyl trimethyl ammonium bromide, is a kind of quaternary ammonium salt, is a kind of cationic surfactant. High purity quaternary ammonium salts are excellent phase transfer catalysts and play a key role in organic synthesis.
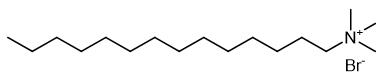
Fig. 1 The structure of Cetrimide.
Physicochemical property
Cetrimide is a white fine powder crystal with a melting point of 245 °C to 250 °C. A rough estimate of its density is 1.1328. It is soluble in water with a solubility of 20 g/100 mL.
Synthetic routes
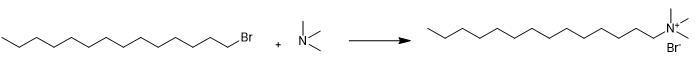
Fig. 2 The synthetic method 1 of Cetrimide.
Add a solution of 1-Bromotetradecane (5 mL, 16.4 mmol) over a trimethylamine (11.7 mL of a solution 4.2 M in ethanol, 49.3 mmol). Stir the reaction mixture at 25 °C under nitrogen atmosphere. Monitor by the TLC technique (hexane/ethyl acetate 1:1). Filter and wash the solid with ethyl acetate (10 mL). Remove the remaining ethyl acetate under reduced pressure. Dry the solid obtained from the reaction under high vacuum for 12 h. 1H NMR (CDCl3, 300 MHz, [ppm]): δ 3.56 (m, 2H, NCH2), 3.47 (s, 9H, N (CH3)3), 1.72 (m, 2H, NCH2CH2), 1.42- 1.18 (m, 22H, N (CH2)2(CH2)11), 0.86 (t, 3H, J= 6.7 Hz, N (CH2)13CH3). 13C NMR (CDCl3, 75.47 MHz, [ppm]): δ 63.7 (NCH2), 51.3 (N (CH3)2), 31.9 (N(CH2)11CH2CH2CH3), 29.6 (3xCH2), 29.4 (2xCH2), 29.3 (2xCH2), 29.2 (CH2), 26.2 (N(CH2)2CH2), 22.7 (NCH2CH2), 22.6 (N(CH2)12CH2), 14.1 (N (CH2)13CH3) [1].
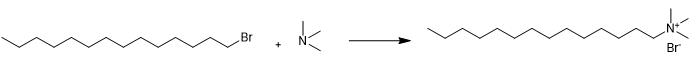
Fig. 3 The synthetic method 2 of Cetrimide.
Add 1-bromotetradecane (0.1 mol) to trimethylamine (0.1 mol) dissolved in 100 mL isopropyl alcohol. Reflux the mixture for 48 hours. Remove isopropyl alcohol by distillation. Evaporate the remaining solvent by using a rotary evaporator. Recrystallize the dried product from absolute alcohol-dry ethyl ether [2].
Application
Antibacterial effect
Chemical sanitizers are commonly used to inactivate Listeria monocytogenes and other Listeria species that persist in food-processing environments after cleaning. In this study, Listeria innocua cultures were exposed to acid, heat, cold, and starvation stress and then assessed for sensitivity to the quaternary ammonium compound cetrimide. Unstressed and stressed cultures were exposed to cetrimide for 3 min, neutralized, and plated on tryptic soy agar with yeast extract to determine the percentage of survivors. Relative to controls, Linnocua exposed to acid and starvation conditions was less sensitive to cetrimide, whereas heat and cold stress increased cetrimide sensitivity (P < 0.05). The diminished sensitivity of acid- and starvation-stressed L innocua to cetrimide suggests that these stressors might increase the persistence of this organism within food-manufacturing facilities. In contrast, enhanced L innocua sensitivity to cetrimide following heat and cold stress suggests that these interventions might increase sanitation efficacy [3].
Effect on root dentin hardness
Introduction: The aim was to investigate the effect of different concentrations of cetrimide with or without 5% EDTA solution on the microhardness of human root dentin in vitro. Methods: Twenty-five recently extracted single-rooted human teeth were selected. The roots were split longitudinally into 2 parts. The specimens were randomly divided into the following 5 groups and were treated with 5% EDTA, 5% EDTA + 0.25% cetrimide, 5% EDTA + 0.50% cetrimide, 0.25% cetrimide, and 0.50% cetrimide immediately after the initial baseline microhardness measurements. A standardized volume of 50 mL of each solution was used for 1 minute. The reference microhardness values of untreated specimens were initially measured with a Vickers indenter under a 50-g load and a 10-second dwell time at the midroot level of the root dentin. Post-treatment microhardness values were obtained in the same manner as the initial ones. The decrease in microhardness was calculated as a percentage. Data were analyzed statistically by 1-way analysis of variance (P = 0.05) and the post hoc Tukey test for multiple comparisons at the same level of significance. Results: All solutions significantly decreased the microhardness of root dentin (P < 0.05). Although there was no significant difference among the solutions (P > 0.05), the specimens in the EDTA + 0.50% cetrimide solution group showed the highest change in microhardness. The plain EDTA and plain 0.50% cetrimide groups had similar values. Conclusions: The use of surfactants higher than 0.25% in concentration is questionable for clinical conditions [4].
Potentiometric PVC membrane Sensor
two types of potentiometric electrodes were constructed for determination of cetrimide. The sensors demonstrated advanced performances with a fast response time, a lower detection limit of 4.0x10-6 M and potential responses across the range of 5.0x10-6-1.0x10-3 M. The sensors enabled the cetrimide measurement in pharmaceutical formulations. The sensors respond based on ion-exchange mechanism. Cetrimide-phosphotungstate showed better and more stable responses in comparison with cetrimide-tetraphenyl borate ion-pair. PVC membrane electrode was made after series of experiments. The best PVC membrane electrode performance was achieved by a membrane composition of 30% PVC, 60% DBP, and 10% ion-pair. The electrode displays a good selectivity for cetrimide with respect to a number of common foreign inorganic and organic species [5].
References
[1] Sintra T E, Vilas M, Martins M, et al. Synthesis and Characterization of Surface‐Active Ionic Liquids Used in the Disruption of Escherichia Coli Cells[J]. ChemPhysChem, 2019, 20(5): 727-735.
[2] Al‐Blewi F F, Al‐Lohedan H A, Rafiquee M Z A, et al. Kinetics of hydrolysis of procaine in aqueous and micellar media[J]. International Journal of Chemical Kinetics, 2013, 45(1): 1-9.
[3] Moorman M, Nettleton W, Ryser E, et al. Altered sensitivity to a quaternary ammonium sanitizer in stressed Listeria innocua[J]. Journal of food protection, 2005, 68(8): 1659-1663.
[4] Akcay I, Sen B H. The effect of surfactant addition to EDTA on microhardness of root dentin[J]. Journal of Endodontics, 2012, 38(5): 704-707.
[5] Faridbod F, Khamseh-Nejad M, Ganjali M R, et al. Cetrimide potentiometric PVC membrane sensor[J]. Int. J. Electrochem. Sci, 2012, 7(3): 1917-1926.
);You may like
See also
Lastest Price from Tetradecyltrimethylammonium bromide manufacturers

US $6.00/KG2024-04-22
- CAS:
- 1119-97-7
- Min. Order:
- 1KG
- Purity:
- More than 99%
- Supply Ability:
- 2000KG/MONTH
US $5.00-0.50/KG2024-03-25
- CAS:
- 1119-97-7
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available