Synthesis and Application of 3,3',4,4'-Biphenyltetracarboxylic dianhydride
General description
The rigid structure of 3,3 ',4,4 '-diphenyltetetrarboxylic anhydride, which is used as raw material to synthesize polyimide is the highest heat resistance resin so far. The polyimide film made by stretching has almost exactly the same thermal expansion coefficient with copper foil, which can be well covered with copper foil under long-term high and low temperature conditions without stripping. Widely used in flexible circuit boards, super power 5G signal transmitter.
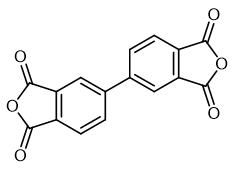
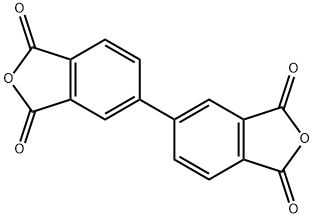
Fig. 1 The structure of 3,3',4,4'-Biphenyltetracarboxylic dianhydride.
Physicochemical property
3,3',4,4' -biphenyltetracarboxylic dianhydride is a white crystalline powder with a melting point of 298-299℃. Its boiling point is expected to be 614.9±48.0 °C and its relative density is expected to be 1.625±0.06 g/cm3.
Synthetic routes
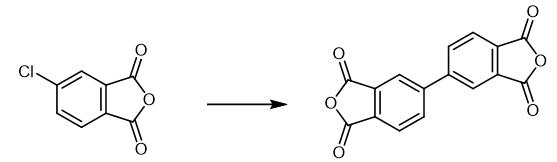
Fig. 2 The synthetic scheme 1 of 3,3',4,4'-Biphenyltetracarboxylic dianhydride.
Add 400.0 g distilled water, 35 g sodium hydroxide (0.875 mol) and 50 g 4-chlorinated anhydride (0.275 mol) into a four-mouth flask equipped with thermometer, agitator, air duct and reflux device, stir it at room temperature for 1 hour. Then 0.303 g of 10 wt% Pd/C recovered from Example 3 and 20 g of compound reducing agent (β-cyclodextrin/serine/xylitol =5/3/2) were added to soak with nitrogen for 30 min, then heated to 90℃ under the protection of nitrogen, the reaction lasted for 12 h, cooled to room temperature and filtered. Drying under nitrogen and recovery palladium carbon catalyst 0.303g (recovery 101%); Add 20 wt% concentrated hydrochloric acid to filtrate to adjust acidity to pH =3; A large amount of white paste precipitate is generated, heated to boiling state under the protection of nitrogen, reflux for 4 hours, cooled to room temperature, cooled to -5°C for 5 hours, and then filtered. Filter cake was washed with deionized cold water for 4 times, dried at 120 °C, heated to 200°C and dehydrated for 4 hours. The crystal powder of 3,3',4,4'-diphenyltetrophthalic anhydride was 77.63 g, melting point 299-301°C, yield 96.33 wt%[1].
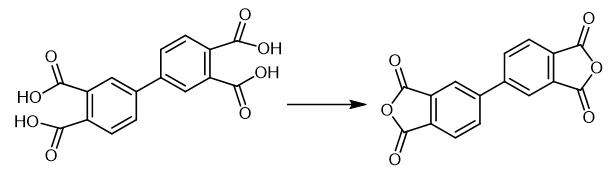
Fig. 3 The synthetic scheme 2 of 3,3',4,4'-Biphenyltetracarboxylic dianhydride.
BTC 100 parts by weight was charged in to a vertical cylindrical reactor equipped with double helical ribbon blade, jacket and inert gas supply port. While stirring, it was heated to 215 under normal pressure and nitrogen gas was circulated at the speed of 2 m3/hr. While purging the water formed, dehydration ring closure reaction was performed for 10 hours. Then, the same reaction container was heated to 300°C and maintained at the same temperature for 5 hours and the reaction material was melted. Melt was transferred to a 1.6 m3 evaporation kettle and maintaining the oxygen concentration in the system to 5 ppm, BPDA was evaporated at 315°C, 230 MPa pressure at the vapour linear velocity of 1.0 m/second. Evaporated BPDA was deposited on the drum type rotation cooler placed at the tip of the gas tube connected directly to the gaseous phase part of the evaporation kettle. The crystals of BPDA adhered to the cooler were scratched continuously scraping device and collected as flakes. These flakes were ground to obtain 80 parts by weight of biphenyl tetracarboxylic acid anhydride (highly pure BPDA). Pd content of the BPDA obtained was measured and was found to be 0.1 ppm. Optical transmittance was measured at wavelength 400 nm and was found to be 92% [2].
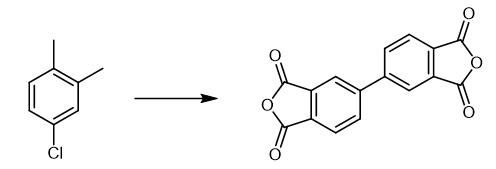
Fig. 3 The synthetic scheme 3 of 3,3',4,4'-Biphenyltetracarboxylic dianhydride.
Iron chloride(III) 1.7 g (0.011 mol; manufactured by Wako Pure Chemical) in tetrahydrofuran solution 6.4 g (0.09 mol) was added, 1,2-dichloropropane 48.2 g (0. 43 mol; manufactured by Wako Pure Chemical) and toluene (manufactured by Wako Pure Chemical) 50 g were added and the catalyst containing solution was prepared. To this, while maintaining the reaction mixture temperature at 50~60°C, the above Grignard reagent solution was added dropwise and the coupling reaction was carried out. After completion of the dropwise addition, aging was carried out for 3 hours at 60 °C. After completion of the reaction, cooled, to the reaction mixture, 5% aqueous solution of hydrochloric acid 100 g was added dropwise. The mixture was stirred, allowed to stand, the liquid separation was performed and the oil layer was obtained. This oil layer was concentrated, methanol 75 g was added, cooling and crystallization were carried out at 5 °C. After solid-liquid separation, the obtained cake was dried in vacuum at 60 °C and biphenyl-3,4,3',4'-tetramethyl 25.9 g (chemical purity 98.3%) was obtained (yield 68.2%). Next, the above obtained biphenyl-3,4,3',4'-tetramethyl 25.9 g was poured into the mixture of tertiary butanol 154.7 g (made by Wako Pure Chem) and water 157.5 g and the temperature was raised to 70 °C. To this, potassium permanganate 187.8 g was added in small portions over 3 hours and aged for 3 hours at 78 °C. The reaction slurry solution was obtained. To this, sodium subsulfite 0.3 g was added and unreacted potassium permanganate was deactivated. This was cooled to 60 °C, generated manganese dioxide was filtered by filtration and the reaction mixture was obtained. Filtered 2 times with warm water 50 ml at 60 °C, the manganese dioxide was rinsed and mixed with the reaction mixture. This solution was concentrated, cooled to room temperature, 35% aqueous solution of hydrochloric acid was added and the value of pH was adjusted to 1.0, biphenyl-3,4,3',4'-tetracarboxylic acid was crystallized and the mixture was stirred overnight. After that, 35% aqueous solution of hydrochloric acid was added and the value of pH was readjusted to 1.0, cooled to 5 °C, filtered, the liquid slurry of biphenyl-3,4,3',4'-tetracarboxylic acid was obtained, after the implementation, rinsed 2 times with cold water 50 ml, dried in vacuum at 60 °C and biphenyl-3,4,3',4'-tetracarboxylic acid 30.8 g (yield 77.0%) was obtained. 30.8 g of biphenyl-3,4,3',4'-7-tetracarboxylic acid obtained in Example 1 and acetic anhydride 6.4 g(0.75 mol) were added and treated for 3 hours at 85 °C, cooled to 5 °C and the solidliquid separation was carried out. The obtained cake was dried under vacuum at 100 °C for 3 hours was carried out and biphenyl-3,4,3',4'-tetracarboxylic acid dianhydride 25.8 g (yield 94.1%) was obtained [3].
Application
Synthesis of polyimide/organic clay nanocomposites
Having previously demonstrated that the polyimide derived from 3,3',4,4'-biphenyltetracarboxylic dianhydride (BPDA) and 1,2-bis(4-aminophenoxy)benzene [termed triphenyl ether catechol diamine (TPEC)] exhibited superior tensile properties in addition to good thermal properties, we now provide a preliminary assessment of the properties of the copolyimides prepared from BPDA, TPEC, and another aromatic diamine. The homopolyimides derived from BPDA and many aromatic diamines generally possessed good mechanical properties and thermal properties; );
You may like
See also
Lastest Price from 3,3',4,4'-Biphenyltetracarboxylic dianhydride manufacturers

US $30.00/kg2024-04-30
- CAS:
- 2420-87-3
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000kg

US $0.00/kg2024-04-12
- CAS:
- 2420-87-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton