Prucalopride Succinate: Pharmacodynamics, Pharmacokinetic Properties and Adverse Effects
General Description
Prucalopride Succinate is a selective serotonin receptor agonist, distinctively designed to treat chronic constipation by enhancing gastrointestinal motility. Approved by the European Medicines Agency in 2009, it demonstrates high affinity for 5-HT4 receptors, promoting colonic contractions and reducing transit time, thereby improving symptoms of constipation. It has favorable pharmacokinetic properties, including rapid absorption, high bioavailability, and minimal metabolism, facilitating once-daily dosing. Adverse effects are generally mild, with the most common being headache, nausea, diarrhea, and abdominal pain, mainly during initial treatment days. Despite its safety profile, rare cases like acute renal failure require cautious monitoring, especially in vulnerable populations.

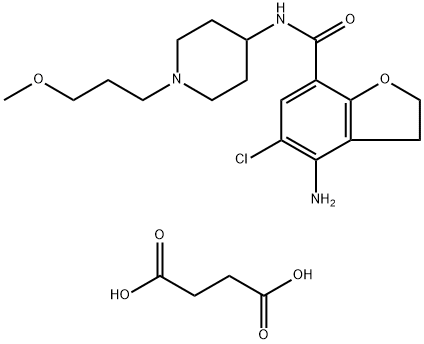
Figure 1. Prucalopride Succinate
Pharmacodynamics
Prucalopride Succinate is a selective serotonin (5-HT4) receptor agonist with a distinct mechanism of action aimed at treating chronic constipation (CC). Approved by the European Medicines Agency in October 2009, it demonstrates a high affinity for 5-HT4 receptors, unlike its predecessors, cisapride and tegaserod, which were linked to cardiovascular risks due to their nonselective prokinetic properties. Prucalopride Succinate enhances gastrointestinal motility and secretion by stimulating cholinergic and non-cholinergic neurotransmission within enteric neurons. This activation leads to the contraction of colonic longitudinal smooth muscles through the release of acetylcholine and substance P, while simultaneously causing relaxation of circular smooth muscles via inhibitory neuromediators like nitric oxide. Its effectiveness extends to inducing giant migrating contractions, akin to high-amplitude propagated contractions in humans, crucial for the propulsion of colonic contents. Clinical trials have demonstrated Prucalopride Succinate's ability to significantly reduce colonic transit time, correlating with an improvement in symptoms of chronic constipation. Additionally, Prucalopride Succinate may possess nociceptive properties, offering potential relief from abdominal pain associated with CC. 1
Pharmacokinetic Properties
Prucalopride Succinate exhibits favorable pharmacokinetic properties that enhance its effectiveness in treating chronic constipation. Upon oral administration, it is rapidly absorbed from the gastrointestinal tract, boasting a high bioavailability exceeding 93%, unaffected by food intake. This allows for flexible dosing schedules, although morning administration is often recommended to synergize with natural colonic motility reflexes. Peak plasma concentrations are achieved within 2 to 3 hours, and the drug maintains a plasma terminal half-life of approximately 30 hours. A steady state is reached after 4 to 7 days of consistent use, with plasma concentration proportionally increasing with the dose. The therapeutic effects of Prucalopride Succinate manifest quickly, with the first spontaneous bowel movement occurring after a median of 2.5 hours, and the first complete spontaneous bowel movement within 50 to 54 hours. The drug undergoes minimal metabolism in humans, with 60% excreted unchanged in urine and 6% in feces, supporting once-daily dosing. Adjustments are recommended for elderly patients over 65 or those with severe renal insufficiency, due to increased plasma concentrations. Notably, Prucalopride Succinate's lack of significant interaction with the cytochrome P450 system and low plasma protein binding minimize drug-drug interactions, allowing concurrent use with other laxatives of different mechanisms, such as osmotics and softeners. 2
Adverse Effects
Prucalopride Succinate is recognized for its high selectivity as a serotonin receptor agonist, primarily targeting the human 5HT4 receptors. This specificity minimizes the cardiovascular adverse effects often associated with less selective serotonin receptor agonists. Unlike other drugs in its class, Prucalopride Succinate does not interact with the hERG potassium channel or the 5-HT2B receptors, contributing to its safety profile. The most common adverse effects reported include headache, experienced by 25-30% of users, nausea (12-24%), diarrhea (12-19%), and abdominal pain (16-23%). These side effects are predominantly observed during the initial days of treatment and do not show significant variation between elderly and non-elderly patients. Notably, Prucalopride Succinate's treatment has demonstrated no significant changes in hemodynamics or ECG readings, including QTc interval prolongation. However, there has been a rare case of acute renal failure after four months of treatment in an elderly patient, suggesting a potential link to tubular necrosis secondary to acute interstitial nephritis. This indicates that while Prucalopride Succinate generally has a favorable safety profile, exceptions exist, warranting cautious monitoring in specific patient populations. 3
Reference
1. Shin A, Camilleri M, Kolar G, et al Systematic review with meta-analysis: highly selective 5-HT4 agonists (prucalopride, velusetrag or naronapride) in chronic constipation. Aliment Pharmacol Ther. 2014; 39: 239–253.
2. Flach S, Scarfe G, Dragone J, et al. A Phase I Study to Investigate the Absorption, Pharmacokinetics, and Excretion of [(14)C]Prucalopride After a Single Oral Dose in Healthy Volunteers. Clin Ther. 2016; 38(9): 2106-2115.
3. Bassotti G, Gambaccini D, Bellini M. Prucalopride succinate for the treatment of constipation: an update. Expert Rev Gastroenterol Hepatol. 2016; 10(3): 291-300.
);You may like
Related articles And Qustion
Lastest Price from Prucalopride Succinate manufacturers
US $0.00/kg2023-11-01
- CAS:
- 179474-85-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 500kg

US $15.00-10.00/KG2021-07-13
- CAS:
- 179474-85-2
- Min. Order:
- 1KG
- Purity:
- 99%+ HPLC
- Supply Ability:
- Monthly supply of 1 ton