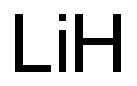PHYSICAL PROPERTIES OF LITHIUM METAL
HISTORY
Davy and Brande independently prepared lithium metal in 1818 by the electrolysis of lithium oxide. However, larger quantities of the metal were prepared by Bunsen and Mattiessen in 1855 by electrolysis of the chloride. Guntz first proposed the preparation of lithium metal by the electrolysis of a fused lithium chloride-potassium chloride mixture (1893)1 0 . Such a process is used to prepare lithium metal commercially today. The first commercial production of lithium metal was carried out in 1923 by Metallgesellschaft, AG, in Germany. From such beginnings the total production of lithium metal has risen to an estimated one million pounds per year.

PRODUCTION
Lithium metal is prepared by the electrolysis of a fused mixture of lithium chloride and potassium chloride. The half reactions occurring in the cell are:
(1) cathode, Li++e-> Li°
(2) anode, Cl~ → 1/2 Cl 2 + e-
The lithium-metal cell is constructed of a low-carbon steel shell, which acts as a cathode and container of the fused salt, and graphite rod anodes. The cell is initially charged with a mixture of about 55 % lithium chloride and 45 % potassium chloride. The electrolyte mixture melts at about 400°C, which is considerably lower than the 610°C melting point of lithium chloride alone. Auxiliary heaters are used to begin melting the electrolyte to start electrical conduction. Once conduction begins, the heat generated by the internal resistance of the cell is sufficient to keep the electrolyte melted. Lithium reduced at the steel cathode floats to the top of the cell where it is skimmed off the electrolyte. Chlorine is released at the anode and is vented. Lithium chloride is added to the cell to replace that which is electrolyzed. Under normal conditions a cell may be operated for several months without shutdown for servicing. The cell operates at an applied potential of 6.0-6.5 V.
CHEMICAL PROPERTIES AND CHEMISTRY
Properties of lithium are given in Table 1. Lithium metal is highly reactive. It reacts with the non-metals, inert gases excepted, most of the metalloids, and many of the metals under proper conditions.

Lithium reacts with the halogens to form lithium halides although it reacts somewhat less readily than do the other alkali metals.
Very pure lithium does not react readily with dry oxygen at room temperature. At 100°C or higher, however, the elements react to form the normal lithium oxide, L i 20. Sodium forms the peroxide under similar conditions. The congeners of oxygen form compounds with lithium at higher temperatures.
Dry nitrogen reacts with lithium only slowly, if at all, at room temperature. However, if a trace of water is present in the nitrogen, lithium nitride, Li3N, is formed even at room temperature. After initiation has taken place, the reaction proceeds readily without the presence of water. The other group Vb elements react with lithium at higher temperatures. Lithium reacts with carbon at elevated temperatures to form lithium carbide, Li2C2, an acetylide.
The reaction of lithium with hydrogen occurs readily at about the melting point of lithium to yield lithium hydride, LiH. The compound is thermodynamically more stable than the hydrides of the other group la metals.
NUCLEAR PROPERTIES
Exposure of a bright surface of lithium metal to moist air results in a rapid tarnishing of the metal. The surface turns black at first and white after longer exposure. Compounds formed are lithium nitride, lithium hydroxide, lithium hydroxide monohydrate and— eventually—lithium carbonate1 1 . Lithium burns in air at high temperatures. The reaction is extremely vigorous, hazardous and difficult to extinguish once ignition occurs. The reaction releases clouds of dense white smoke, presumably containing lithium oxide.
Large pieces of lithium react rapidly with water, evolving hydrogen and forming a solution of lithium hydroxide. The reaction is not as vigorous as the reaction between sodium and water, perhaps in part because lithium does not melt during the reaction as does sodium. As with the other alkali metals, powdered lithium reacts with water with explosive violence.
PHYSICAL PROPERTIES
Several physical properties of lithium metal are given in Table 1. One of the most interesting properties is the extremely low density, 0.534 g c m - 3 . At normal temperatures lithium has the lowest density of the non-gaseous elements. The metal is harder and has a higher melting point than the other alkali metals.
Above — 117°C, lithium metal has a body-centered cubic crystal structure typical of the alkali metals. On cooling to — 201 °C, the metal begins to transform to the face-centered cubic structure. Further cooling causes a greater degree of transformation, but complete conversion does not occur. Cold working causes a transformation from the body-centered cubic to the face-centered cubic structure at somewhat higher temperatures 1 2 . The crystal lattice constant is 3.50 A at 20° C13 .
Lithium forms an equilibrium mixture of monatomic and diatomic molecules on vaporization. The heat of dissociation of the dimer is 25.76 + 0.10 kcal mole- 1 at 0° K14 .
NUCLEAR PROPERTIES
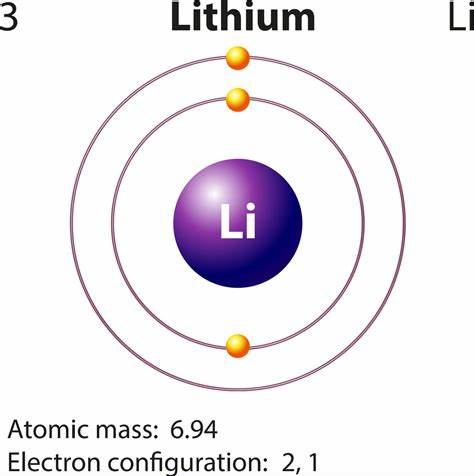
Lithium has two naturally occurring isotopes, 6Li and 7Li. The natural abundance of Li is 7.42%. The natural abundance of 7Li is 92.58 %. The thermal neutron capture crosssection for 6Li is 910 barns; for 7Li, 0.33 barns.
At least three other nuclides have been artificially produced: 5Li, 8Li and 9Li. They are unstable, decaying by the following modes:
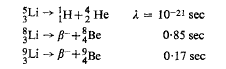
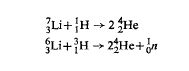
Lithium isotopes undergo the following thermonuclear reactions which release large amounts of energy 15.