Pharmacokinetics and Toxicity of Biapenem
Mar 9,2022
DESCRIPTION
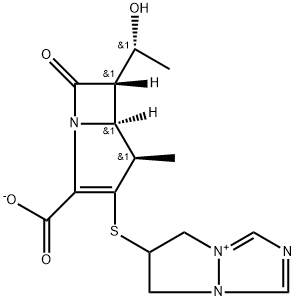
Biapenem is a parenteral carbapenem antibiotic that possesses antibacterial activities against a wide range of Gram-positive and -negative bacteria. The introduction of the methyl radical at the 1-b position of the carbapenem skeleton gives biapenem stability against hydrolysis by renal dehydropeptidase-I. Therefore, biapenem does not require concomitant administration of an inhibitor of this enzyme. The molecular formula for biapenem is C15H18N4O4S and its molecular weight is 350.39. Biapenem is not currently licensed for use, and its role compared with other available carbapenems such as imipenem and meropenem is currently unclear.Anaerobic bacteria
Biapenem inhibits both Gram-positive and Gram-negative anaerobic bacteria. Some Bacteroides fragilis strains that produce metallo-b-lactamase hydrolyze carbapenems, including biapenem – the amount of enzyme production correlates with the MIC.MECHANISM OF DRUG ACTION
The mode of action of biapenem is similar to that of imipenem and meropenem. In S. aureus and E. coli, binding of biapenem to PBP shows a similar pattern with that of imipenem and meropenem. Biapenem is a parenteral carbapenem that is stable against renal dehydropeptidase-I. Therefore, it does not require the coadministration of a renal dehydropeptidase-I inhibitor.PHARMACOKINETICS AND PHARMACODYNAMICS
Biapenem is only available intravenously. When biapenem is administered as a 300-mg intravenous infusion over 30 minutes to healthy volunteers, a peak plasma concentration of about 19 mg/ml is reached 30 minutes after the end of the infusion. Eight hours after the infusion of biapenem, the plasma concentration level is 0.2 mg/ml. The serum half-life of the drug was approximately 1 hour. After single doses of 0.3 or 0.6 g, AUC is approximately 30 and 55 mg/ml per hour, respectively. Age-related changes were observed in AUC and renal clearance of biapenem. When 300 and 600 mg were administered to ten healthy elderly volunteers mean AUC (44.6 and 91.9 mg/ml per hour) was significantly higher than that in the five healthy young volunteers (26.6 and 66.1 mg/ml per hour). Biapenem displays very low serum protein binding of about 7% .TOXICITY
The most common adverse events in a clinical study for adult pneumonia, chronic respiratory tract infection, and complicated urinary tract infection patients were rash and diarrhea. Abnormal clinical laboratory tests were observed in 14.8–29.5% patients. Abnormal laboratory results consisted mostly of elevated eosinophil counts and liver function tests, including alanine aminotransferase and aspartate aminotransferase and were transient.In a study with 316 pediatric patients, four cases of rash, four cases of diarrhea, and one case each of erythema, stomachache, and fever were observed. Abnormal clinical laboratory tests were observed in 14.7%, and most abnormalities consisted of transient elevation of eosinophil, platelet, and transaminase (Fujii et al., 1994). Animal studies have shown that biapenem is less likely to induce convulsions than imipenem or imipenem–cilastatin and as likely with meropenem.CLINICAL USES OF THE DRUG
Biapenem is not currently licensed for use. However, it has been assessed for use in a number of clinical situations, including intra-abdominal sepsis and pneumonia. The clinical role for biapenem in comparison with other available carbapenems, such as imipenem and meropenem, is currently unclear.In a Swedish study in which 43 patients with complicated intra-abdominal infection received biapenem 500 mg every 8 hours intravenously, 28 patients (62.7%) were clinically cured and the microbiologic response was satisfactory in 28 of 43 patients (65.1%).In Japanese trials, for the treatment of patients with chronic lower respiratory tract infection and bacterial pneumonia, biapenem showed clinical and bacteriologic efficacy similar to that of imipenem.In studies of the efficacy of biapenem 150 and 300 mg twice daily given for up to 14 days, the clinical efficacy was 100% (ten of ten patients) and 90% (nine of ten patients), respectively.In Japan, biapenem is used for sepsis, lower respiratory infection, complicated urinary tract infection, peritonitis, and intrapelvic infection on gynecology. In the treatment of complicated urinary tract infection, patients receive biapenem 300 mg twice daily for 5 days. Clinical efficacy of biapenem was 94.7% (71 of 75 patients) compared with the imipenem–cilastatin group (93.4%).);You may like
Lastest Price from Biapenem manufacturers
Biapenem

US $20.00/kg2024-02-27
- CAS:
- 120410-24-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 Ton
Biapenem

US $30.00-20.00/kg2024-01-08
- CAS:
- 120410-24-4
- Min. Order:
- 1kg
- Purity:
- 99.50%
- Supply Ability:
- 20tons