Paliperidone: A Safe and Effective Treatment Option for Schizophrenia and Schizoaffective Disorder
General Description
Paliperidone, an atypical antipsychotic, is available in oral and long-acting injectable forms for treating schizophrenia in adults and adolescents. Its mechanism of action involves dopamine and serotonin receptor antagonism, as well as effects on adrenergic and histaminergic receptors. Studies have shown its efficacy in reducing symptoms and delaying relapse, with a generally well-tolerated safety profile. Particularly beneficial for elderly patients and those with treatment noncompliance, it offers potential neuroprotective effects and improved cognitive outcomes. Overall, the extensive research supports paliperidone as a safe and effective treatment option for schizophrenia and schizoaffective disorder, with benefits in symptom reduction, relapse prevention, and cognitive improvement.

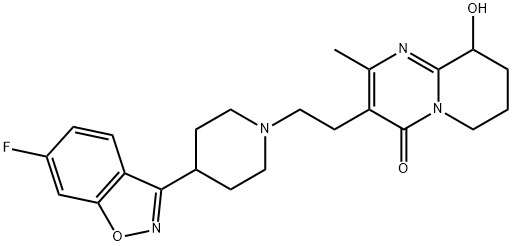
Figure 1. Paliperidone
Overview
Paliperidone is an atypical antipsychotic approved for use in the US in 2006, available in daily oral extended-release tablets and 1-month or 3-month LAI formulations. The extended-release form is indicated for treating schizophrenia in adults and adolescents ages 12–17, with available dosages of 1.5 mg, 3 mg, 6 mg, 9 mg, and 12 mg. The LAI form is indicated for schizophrenia or schizoaffective disorder in adults as monotherapy or in conjunction with mood stabilizers, with the 1-month LAI available in dosages of 39 mg, 78 mg, 117 mg, 156 mg, or 234 mg, and the 3-month injectable in dosages of 273 mg, 410 mg, 546 mg, or 819 mg. Contraindications include hypersensitivity reactions to paliperidone or risperidone, and its use is not recommended for elderly patients with dementia-related psychosis due to increased mortality risk. Adverse events such as cerebrovascular disease, neuroleptic malignant syndrome, QT prolongation, extrapyramidal symptoms, weight gain, dyslipidemia, hyperglycemia, and cognitive impairment may occur. 1
Mechanism of Action
Paliperidone, a member of the benzisoxazole derivative class of atypical antipsychotics, exerts its mechanism of action through a multifaceted approach. Like other atypical antipsychotics, paliperidone demonstrates affinity for dopamine D2 and serotonin 5-HT2A receptors, with a higher ratio of antagonism at 5-HT2A than D2 receptors. It also exhibits antagonistic effects at α1 and α2 adrenergic and H1 histaminergic receptors, while lacking affinity for M1 cholinergic or β adrenergic receptors. Positron emission tomography studies have revealed that paliperidone occupies a significant proportion of D2 receptors in specific brain regions, with distinct differences from its precursor risperidone in terms of receptor affinities. Furthermore, paliperidone's receptor antagonism has been shown to impact serotonergic and noradrenergic signaling differently than risperidone in animal models, and it induces changes in mitochondrial protein expression in the prefrontal cortex analogous to those seen with lithium, suggesting potential mood stabilizing properties. In addition, molecular signaling events and protein expression changes associated with paliperidone's receptor binding suggest modulation of neurotransmitter release, synaptic plasticity, and receptor signaling, indicating a complex and nuanced mechanism of action. Overall, paliperidone's unique receptor affinities and downstream effects differentiate it from other atypical antipsychotics and may contribute to its clinical efficacy. 2
Safety and Efficacy
Paliperidone is an antipsychotic medication that has been extensively studied for its safety and efficacy in managing schizophrenia and schizoaffective disorder. Numerous studies have utilized various measures to assess the efficacy of paliperidone, including the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression Severity (CGI-S) Scale, Young Mania Rating Scale (YMRS), Hamilton Depression Rating Scale 21-item version (HDRS-21), and the Personal and Social Performance (PSP) Scale. These studies consistently demonstrate that paliperidone effectively reduces symptoms and delays relapse in patients with schizophrenia. In terms of safety, paliperidone is generally well-tolerated; however, some common adverse effects include headache, anxiety, insomnia, weight gain, suicidal ideation, nasopharyngitis, urinary tract infections, and extrapyramidal symptoms. Studies have also compared the efficacy of paliperidone with placebo and other antipsychotic medications, consistently showing superior results in reducing relapse rates and maintaining cognitive functioning. Special attention has been given to the use of paliperidone in elderly patients and those with treatment noncompliance. Paliperidone has been found to be particularly beneficial for elderly patients due to its reduced susceptibility to changes in metabolism and lower incidence of adverse effects related to motor function. Furthermore, long-acting formulations of paliperidone, such as paliperidone palmitate, have shown promise in improving compliance and reducing relapse rates in patients with chronic noncompliance issues. Additionally, recent research has explored the potential neuroprotective effects of paliperidone, indicating that it may lead to faster cognitive improvements compared to other antipsychotic medications. Studies have also investigated the use of paliperidone in long-acting formulations administered once every three months, demonstrating similar efficacy and safety profiles compared to the more conventional monthly injections. In summary, the extensive body of research supports the conclusion that paliperidone is a safe and effective treatment option for patients with schizophrenia and schizoaffective disorder, offering benefits in symptom reduction, relapse prevention, and cognitive improvement. 3
Reference
1. FDA INVEGA SUSTENNA? (Paliperidone Palmitate) Extended-Release Injectable Suspension, for Intramuscular Use. [(accessed on 17 November 2020)];2019.
2. Corena-McLeod M. Comparative Pharmacology of Risperidone and Paliperidone. Drugs R D. 2015;15(2):163-174.
3. Minwalla HD, Wrzesinski P, Desforges A, et al. Paliperidone to Treat Psychotic Disorders. Neurol Int. 2021;13(3):343-358.
You may like
Related articles And Qustion
Lastest Price from Paliperidone manufacturers

US $0.00/kg2025-05-16
- CAS:
- 144598-75-4
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $0.00-0.00/Kg/Bag2025-04-21
- CAS:
- 144598-75-4
- Min. Order:
- 1Kg/Bag
- Purity:
- 0.99
- Supply Ability:
- 20 tons