Nonafluorobutanesulfonyl fluoride: Characteristic, Applications in Domino Reaction and Neurotoxicity
General Description
Nonafluorobutanesulfonyl fluoride is a versatile reagent with stable and highly reactive characteristics, commonly used in the synthesis of naphthols and as a key component in domino reaction processes. Its unique ability to release fluoride anions during reactions with fatty alcohols enables efficient fluorination processes. However, Nonafluorobutanesulfonyl fluoride has been associated with neurotoxicity, impacting neurobehavior and molecular mechanisms in zebrafish embryos. Comparative studies with perfluorooctanesulfonic acid (PFOS) revealed similar dysregulated pathways related to oxidative stress and lipid metabolism. While less toxic than PFOS, Nonafluorobutanesulfonyl fluoride still poses neurotoxic risks and necessitates careful handling and disposal to mitigate potential harm. Understanding these dual aspects of Nonafluorobutanesulfonyl fluoride is crucial for maximizing its benefits in organic synthesis while minimizing its environmental and health impacts.

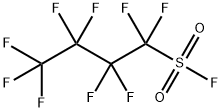
Figure 1. Nonafluorobutanesulfonyl fluoride
Characteristic
Nonafluorobutanesulfonyl fluoride is a useful and cost-effective reagent that is stable and has excellent reactivity with phenolic hydroxyl groups. It is commonly used as a reactant in the synthesis of naphthols. When reacting with fatty alcohols, Nonafluorobutanesulfonyl fluoride releases fluoride anions that will attack the back of the neighboring benzoic acid salt (NfO) in a nucleophilic substitution reaction, resulting in a complete fluorination process. This unique characteristic of Nonafluorobutanesulfonyl fluoride makes it an excellent reagent for the efficient conversion of alcohols. Although there have been numerous reports on the use of Nonafluorobutanesulfonyl fluoride for the synthesis of naphthols through the phenolic hydroxylation reaction, prior to the author's report, there was no effective utilization of the generated fluoride anions in the reaction. This highlights the importance of understanding the unique characteristics of Nonafluorobutanesulfonyl fluoride in order to fully leverage its reactivity and achieve optimal results in organic synthesis. Overall, Nonafluorobutanesulfonyl fluoride's stability and excellent reactivity make it a valuable tool in various chemical reactions and organic synthesis processes. 1
Applications in domino reaction
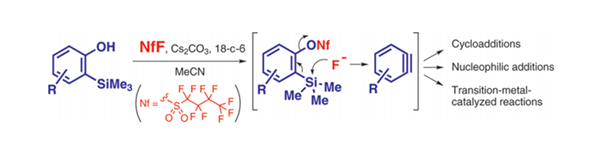
Nonafluorobutanesulfonyl fluoride is utilized in the conversion of stable 2-(trimethylsilyl)phenyl trimethylsilyl ethers to benzynes, serving as a key component in a domino reaction process. This compound plays a crucial role in the transformation of the ethers into benzynes through a series of steps involving O-desilylation, O-nonaflylation, and β-elimination under mild conditions. Together with tetrabutylammonium triphenyldifluorosilicate (TBAT), Nonafluorobutanesulfonyl fluoride facilitates the generation of benzynes, which are highly reactive intermediates. These benzynes are subsequently captured by various arynophiles, resulting in the formation of a diverse range of benzo-fused heterocycles. The application of Nonafluorobutanesulfonyl fluoride in this process enables the efficient synthesis of complex molecular structures with significant potential in organic synthesis and pharmaceutical research. 2
Neurotoxicity
Nonafluorobutanesulfonyl fluoride is a chemical compound that has been implicated in neurotoxicity. While the exact mechanism of NfF-induced neurotoxicity is not fully understood, studies have shown that exposure to Nonafluorobutanesulfonyl fluoride can lead to altered neurobehavior patterns and affect endogenous neurochemicals in zebrafish embryos. In a recent study, the neurotoxicity of Nonafluorobutanesulfonyl fluoride was compared to that of perfluorooctanesulfonic acid using integrative multi-omics analysis in zebrafish embryos. Despite being approximately 700 times less toxic than PFOS, Nonafluorobutanesulfonyl fluoride exhibited similar dysregulated molecular mechanisms associated with oxidative stress, lipid metabolism, and glycolysis/glucogenesis. The study also found commonalities in the developmental neurotoxicity-related mechanisms between Nonafluorobutanesulfonyl fluoride and PFOS, which included the calcium signaling pathway, lipid homeostasis, and primary bile acid biosynthesis. These findings suggest that while NfF may be less toxic than PFOS, it still exhibits similar neurotoxic effects and should be carefully managed to minimize potential toxicity. Further research is needed to fully understand the neurotoxicity mechanism of Nonafluorobutanesulfonyl fluoride and to develop strategies for safe handling and disposal of this compound. Overall, this study highlights the importance of considering the potential neurotoxicity of industrial chemicals like Nonafluorobutanesulfonyl fluoride when evaluating their safety for human and environmental exposure. 3
Reference
1. Ikawa T. Efficient synthesis of multisubstituted aromatic compounds from phenol derivatives. Yakugaku Zasshi. 2014;134(12):1309-1317
2. Ikawa T, Masuda S, Nakajima H, Akai S. 2-(Trimethylsilyl)phenyl Trimethylsilyl Ethers as Stable and Readily Accessible Benzyne Precursors. J Org Chem. 2017;82(8):4242-4253.
3. Min EK, Lee H, Sung EJ, et al. Integrative multi-omics reveals analogous developmental neurotoxicity mechanisms between perfluorobutanesulfonic acid and perfluorooctanesulfonic acid in zebrafish. J Hazard Mater. 2023;457:131714.
);You may like
Related articles And Qustion
Lastest Price from Nonafluorobutanesulfonyl fluoride manufacturers

US $50.00/kg2024-04-26
- CAS:
- 375-72-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000

US $120.00/kg2024-04-25
- CAS:
- 375-72-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20ton