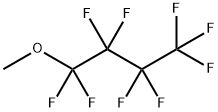
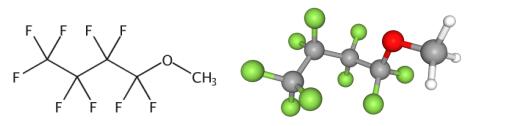
Methyl Nonafluorobutyl Ether: Green Preparation and Applications in Lithium Batteries
General Description
The green synthesis of Methyl Nonafluorobutyl Ether offers an eco-friendly method for producing perfluoroalkyl ethers, using potassium hydroxide and fluoride as catalysts to react perfluorobutyryl fluoride with a carbonate alkylating agent. This process, avoiding organic solvents, unfolds in two phases: a low-temperature reaction followed by a high-temperature phase, emphasizing sustainability and resulting in a non-toxic product suitable for cleaning and insulation. Additionally, Methyl Nonafluorobutyl Ether enhances lithium battery safety by serving as a nonflammable electrolyte component when mixed with conventional solvents and lithium salts. The addition of cyclic carbonates and Methyl Nonafluorobutyl Ether improves interfacial resistance, charge-discharge efficiency, and cycle life, showcasing its potential in advancing battery technology while aligning with environmental objectives.

Figure 1. Methyl nonafluorobutyl ether
Green Preparation
The green preparation of Methyl Nonafluorobutyl Ether represents an environmentally friendly approach to synthesizing perfluoroalkyl ethers, crucial for various industrial applications. This method leverages potassium hydroxide and potassium fluoride as catalysts to facilitate the reaction between perfluorobutyryl fluoride and a carbonate alkylating agent. The process unfolds in two main stages: an initial reaction phase at low temperatures around -20°C for 2 to 4 hours, followed by a higher temperature phase between 200°C to 250°C for 9 to 10 hours. A distinctive feature of this green synthesis is the elimination of organic solvents, which are often associated with environmental and health hazards. Instead, the use of carbonate as an alkylating agent not only reduces the toxicity of the process but also simplifies the handling of the final product. This methodological choice underscores a commitment to sustainability, aligning with broader environmental objectives.The resultant Methyl Nonafluorobutyl Ether is characterized by its non-toxic nature, making it suitable for applications as a cleaning agent and insulating fluid. The simplicity of the process, coupled with the clarity and ease of purification of the product, highlights the practical advantages of this green preparation technique. By adopting such environmentally conscious manufacturing practices, the production of Methyl Nonafluorobutyl Ether contributes to safer and more sustainable chemical processes within the industry. 1
Applications in Lithium Batteries
Methyl Nonafluorobutyl Ether represents a significant advancement in the development of safer electrolytes for lithium secondary batteries, primarily due to its nonflammable properties. Its integration into battery technology aims to enhance safety without compromising performance. By mixing Methyl Nonafluorobutyl Ether with conventional electrolyte solvents like dimethyl carbonate or ethyl methyl carbonate (EMC), and dissolving organic lithium salts such as Li[SO2C2F5]2 (LiBETI) into this blend, a no-flash-point (NFP) electrolyte solution is created. This innovative approach addresses the flammability concerns associated with traditional electrolytes, thereby contributing to the overall safety of lithium batteries. Research indicates that the performance of lithium batteries incorporating Methyl Nonafluorobutyl Ether-based NFP electrolyte can be further optimized by adjusting the solid electrolyte interface (SEI) film on the graphite anode. This optimization involves adding cyclic carbonates, such as ethylene carbonate (EC), and lithium hexafluorophosphate (LiPF6) to the electrolyte mix. These additives have been shown to significantly reduce interfacial resistance, enhancing both the charge-discharge efficiency and the cycle life of the batteries. For instance, a 18650 cylindrical cell with EC and LiPF6 added to the LiBETI-Methyl Nonafluorobutyl Ether/EMC electrolyte demonstrated a discharge capacity above 90% at a 1C current rate and maintained over 80% of its initial capacity after 560 cycles at room temperature. The effectiveness of these additives in improving battery performance has been thoroughly investigated through various analytical techniques, including electrochemical spectroscopy, X-ray photoelectron spectroscopy, solid-state 7Li nuclear magnetic resonance, and attenuated total reflection infrared spectroscopy. These studies underscore the potential of Methyl Nonafluorobutyl Ether in creating safer, more efficient lithium batteries, marking a significant step forward in battery technology. 2
Reference
1. Chen J, Hou YD, Xu B, Cheng X, Yang BX, Gu TH. Green preparation and application of perfluoroalkyl ether. 2022. Patent Number: CN114853578.
2. Arai J. Nonflammable Methyl Nonafluorobutyl Ether for Electrolyte Used in Lithium Secondary Batteries. Journal of The Electrochemical Society. 2003; 150: 1.
);You may like
Related articles And Qustion
Lastest Price from METHYL NONAFLUOROBUTYL ETHER manufacturers

US $10.00/PCS2024-05-28
- CAS:
- 163702-07-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 mt
US $0.00-0.00/KG2024-03-25
- CAS:
- 163702-07-6
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- kg-tons