Methenolone acetate - Pharmacokinetics, Synthesis, Safety etc.
Primobolan is a DEA Schedule III controlled substance. Substances in the DEA Schedule III have a potential for abuse less than substances in Schedules I or II and abuse may lead to moderate or low physical dependence or high psychological dependence.

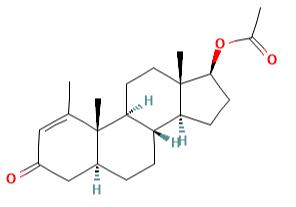
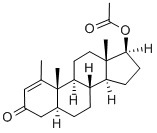
Figure 1 the molecular formula of Methenolone acetate
Pharmacokinetics
The metabolism of methenolone acetate (Primobolan) has been thoroughly investigated in humans owing to its use as a doping agent among athletes. Like many other androgenic-anabolic steroids, it has been used in sports in the last two decades with the intention of increasing strength and improving performance. The use of methenolone acetate is prohibited in sports by the International Olympic Committee because of concerns over the potential incidence of adverse health effects and for ethical reasons. In residue analysis of veterinary medicinal products the use of anabolic steroids has been prohibited in the European Union since 1981.Primobolan is used in the treatment of aplastic anaemia because of its therapeutic efficiency and lower hepatic toxicity comparedto its 17α-alkylated analogues.
Pancytopenia still has a very serious prognosis, which is not significantly affected by methenolone. This makes bone marrow transplantation an attractive alternative in suitable cases. According to our information, methenolone acetate (Primobolan) has become increasingly popular as a doping agent among athletes during the last decade. For doping detection laboratories, methenolone is relatively difficult to assay. In contrast to most other anabolic steroids. methenolone is not an 19-nor steroid and does not contain an alkyl group in l7a-position. Antibodies have been raised towards 17cl-alkylated and 19-nor steroids. and these antibodies do not cross-react with methenolone.[1]
Synthesis
Owing to the 1-position of the double bond of methenolone, the compound has some specific characteristics different from testosterone, which has a double bond in the 4-position. Methenolone is not able to aromatize to estrogens, which leads to a considerably higher anabolic activity than testosterone homologues. Therefore, methenolone has a possible use in breeding animals.[2]
The chemistry of Meldrum’s acid has been surveyed in comprehensive reviews[3]and a micro-1-Methyl-Scr-androst-1-ene-3,17-dione, a kind gift from Dr Schulze, was used as starting material. The compound can be prepared from methenolone by oxidation with chromic acid (Fig. I). 1-Methyl-5a- androst-1-ene-3,17-dione was isomerized to I-methy- len-5a-androstane-3,I ‘I-dione by a general method for conversion of a&unsaturated ketones into P,y-unsaturated ketones described by Ringold and Malhotra[S]. 1-Methyl-5-androst-1-ene-3,17-dione, 20 mg, was dissolved in a solution of potassium tert butanol in tert butanol. The latter solution was obtained by dissolving 25 mg of potassium in 0.5 ml f tert butanol. The mixture was stirred under nitro-gen at room temperature for 24 h. Aqueous acetic acid, 25x, was then added and the mixture was im- mediately extracted with ether. The ether extract was rapidly washed with an aqueous solution of sodium bicarbonate (5%. v/v] and then with water until neu-&at. The ether extract was dried with anhydrous sodium sulfate. The major product obtained had the chromatographic properties expected for 1-methy- len-5r-androstane-3,17-dione when subjected to thin-layer chromatography with toluene-ethyl acetate (4:1, v/v) as solvent. Only traces of unconverted 1-methyl-5r-androst-1-ene-3,17-dione were present. The identity of l-methylen-5a-androstane-3,I7-dione w;ts confirmed by treatment with 0.2 M potassium hy- droxide in aqueous ethanol. As expected for a @,T- unsaturated ketone, this treatment gave a quantitative reconversion into the corresponding a&unsaturated ketone, 1-methyl-5r-androst-1-ene-3,17-dione.[2]

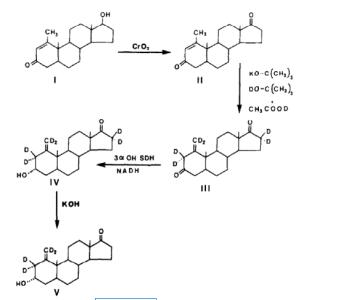
Figure 3 Synthesis of the @, T major urinary metabolite of methenolone acetate
Toxicological and Safety
Owing to the substitution of the C-17 atom, methenolone acetate is protected from rapid
hepatic metabolism and is therefore orally active. When administered orally, methenolone
acetate is six times less androgenic than testosterone propionate and three times more anabolic than orally active 17α-methyltestosterone.[4]
References
1.Lockner D., "Treatment of Refractory Anemias with Methenolone," Acta Medica Scandinavica, Vol.205, No.1‐6(1979), pp.97-101.
2
3.Lavon O. & Bejel S., "Safety of brotizolam in hospitalized patients," European Journal of Clinical Pharmacology, Vol.74, No.7(2018), pp.939-943.
4
);You may like
Related articles And Qustion
See also
Lastest Price from Methenolone acetate manufacturers

US $90.00-70.00/kg2024-05-11
- CAS:
- 434-05-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg

US $9.90-8000.00/g2024-05-11
- CAS:
- 434-05-9
- Min. Order:
- 1g
- Purity:
- 99.99%
- Supply Ability:
- 2 tons