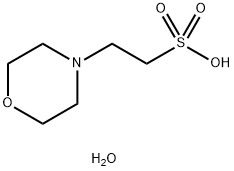
MES Monohydrate: Diverse Applications and Preparation Method
General Description
MES monohydrate is a versatile compound with diverse applications in both buffering and catalysis. As a buffering agent, MES monohydrate is valued for its ability to maintain a stable pH range of 6.0-7.0, making it ideal for biological systems that require a slightly acidic environment. Its low UV absorbance minimizes interference with spectrophotometric measurements, while its minimal temperature dependence ensures reliable buffering capacity across various temperatures. In biochemical and molecular biology research, MES monohydrate serves as a reliable buffer for maintaining pH stability in enzyme assays, cell culture, and protein purification processes. Additionally, MES monohydrate finds application as a water-soluble catalyst in the environmentally friendly synthesis of isoindolo [2,1- a ]quinazolines, offering enhanced reaction efficiency under mild conditions. Overall, MES monohydrate's versatility and effectiveness make it a valuable tool in biological research and organic synthesis, enabling precise control over experimental conditions and promoting high yields of desired products.

Figure 1. MES monohydrate
Diverse Applications
Application as Buffers
MES monohydrate, is a popular buffering agent widely used in biochemical and molecular biology research due to its unique properties. With a pKa of 6.1, MES is effective at maintaining a stable pH around 6.0-7.0, making it particularly suitable for buffering biological systems that require a slightly acidic environment. One key advantage of MES monohydrate as a buffer is its low UV absorbance, which minimizes interference with spectrophotometric measurements commonly used in biological assays. Additionally, MES monohydrate exhibits minimal temperature dependence, providing reliable buffering capacity across a range of temperatures commonly encountered in laboratory settings. Moreover, MES monohydrate is compatible with a variety of biological molecules and enzymatic reactions, making it a versatile choice for maintaining pH stability in various experimental conditions. Its ability to maintain pH without interfering with biochemical reactions makes it an ideal choice for studies requiring precise control over pH levels, such as enzyme assays, cell culture, and protein purification processes. Overall, MES monohydrate serves as a reliable and effective buffering agent in biological research, ensuring accurate and consistent results in diverse experimental settings. 1
Application as catalyst
MES monohydrate is a water-soluble catalyst that has found application in the efficient and environmentally friendly synthesis of isoindolo [2,1- a ]quinazolines. This catalyst enables a one-pot synthesis process at room temperature, with the added benefit of ultrasonication. The combination of MES monohydrate as a catalyst and ultrasonication leads to an excellent yield of the desired product while operating under mild conditions. The use of MES monohydrate as a catalyst offers several advantages. Firstly, its water solubility makes it an attractive option for catalyzing reactions in aqueous environments. Additionally, the catalyst operates at ambient temperature, reducing the energy requirements of the synthesis process. Furthermore, the synergy between MES monohydrate and ultrasonication enhances the efficiency of the reaction, potentially leading to improved reaction kinetics and product yield. Overall, the application of MES monohydrate as a catalyst in the synthesis of isoindolo [2,1- a ]quinazolines demonstrates its potential as a greener alternative for conducting organic transformations with high efficiency and minimal environmental impact. 2
Preparation method
The cost-effective preparation method of MES monohydrate, involves several key steps to ensure high production efficiency and excellent product quality. Firstly, an organic solution containing morpholine and sodium chloroethanesulfonate is mixed and heated under reflux at a temperature range of 60-70 °C. Subsequently, sodium methoxide is slowly added to the reaction system to regulate the pH and facilitate the formation of a methanol solution of 2-morpholine ethanesulfonic acid. Following this, the pH is adjusted to 5 by adding a hydrochloric acid solution and impurities such as sodium chloride are removed through hot filtration, resulting in the formation of frozen crystals of 2-(4-morpholinyl)ethanesulfonic acid solution. These crystals are then subjected to centrifugation to separate and purify the MES monohydrate, ensuring the final product's high purity and superior quality. This method not only ensures cost-effectiveness but also guarantees efficient production of MES monohydrate. 3
Reference
1. 2-(N-Morpholino)ethanesulfonic acid. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 78165.
2. D. BHAGAT. 2-Morpholinoethanesulfonic acid catalyzed one pot synthesis of isoindolo [2,1-a]quinazoline at room temperature under ultrasonication. European Chemical Bulletin. 2015; 4(1): 410-413.
3. Cost-effective preparation method of 2-(4-morpholinyl)ethanesulfonic acid. 2019. Patent Number: CN109134403.
You may like
Related articles And Qustion
See also
Lastest Price from MES monohydrate manufacturers

US $0.00/kg2025-05-16
- CAS:
- 145224-94-8
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20MT

US $0.00/KG2025-04-21
- CAS:
- 145224-94-8
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 3500kg/month