Mebendazole Shows Promising Potential as a Therapeutic Agent for Brain Cancer
General Description
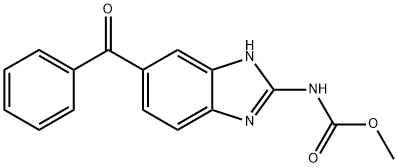
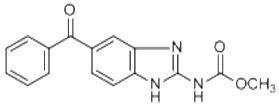
Mebendazole has shown promising preclinical evidence in the treatment of brain cancer by inhibiting tumor cell growth, targeting angiogenesis and Hh signaling, and sensitizing cancer cells to conventional therapies. Clinical evidence suggests that Mebendazole may possess antitumoral properties and could potentially be used as an adjuvant or neoadjuvant therapeutic for cancer treatments. Multiple ongoing clinical trials are investigating the anticancer effects of Mebendazole alone or in combination with other drugs. While further clinical evaluation is still needed, the available evidence supports the potential use of Mebendazole as a therapeutic agent for brain cancer.
Figure 1. Tablets of mebendazole
Preclinical Studies in Brain Cancer
Mebendazole has shown promising preclinical evidence in the treatment of brain cancer. Anthelmintic drugs, including Mebendazole, have gained attention as potential anticancer agents due to their interaction with microtubules. Mebendazole has been found to inhibit tumor cell growth by inhibiting tubulin polymerization, leading to a lethal effect in rapidly dividing cells. Studies have demonstrated the potential of Mebendazole in inhibiting cancer cell growth in various types of cancer, including thyroid, gastrointestinal, breast, prostate, pancreatic, ovarian, colorectal, melanoma, head and neck, leukemia, and bile duct cancer. Mebendazole can also modulate various cancer-associated pathways, depending on the specific cancer model. In the case of brain cancer, several studies have shown the antitumor properties of Mebendazole. It has been found to induce apoptosis in glioblastoma multiforme (GBM) cell lines and significantly extend mean survival in mouse glioma models. Furthermore, the combination of Mebendazole with temozolomide (TMZ) has shown improved survival outcomes compared to TMZ alone. Mebendazole has also demonstrated potential in targeting angiogenesis, a key process in glioma pathology. It can inhibit vascular endothelial growth factor receptor 2 (VEGFR2) autophosphorylation, which plays a crucial role in tumor angiogenesis. Mebendazole selectively targets tumor angiogenesis without affecting normal brain vasculature. Additionally, Mebendazole has shown inhibitory effects on the Hedgehog (Hh) signaling pathway, which is involved in tumor cell growth and invasion. Inhibition of Hh signaling is a validated therapeutic strategy for various cancers, including medulloblastoma. Mebendazole has been found to inhibit Hh signaling and decrease cell proliferation in medulloblastoma cell lines. Mebendazole has also demonstrated the ability to sensitize cancer cells to conventional therapies, such as chemotherapy and radiation, enhancing their combined antitumor potential. It has been shown to increase survival and induce apoptosis in preclinical models of malignant meningioma and glioblastoma when used alone or in combination with radiation. Furthermore, studies have suggested that combining Mebendazole with other chemotherapy drugs, such as temozolomide and vinblastine, may lead to synergistic responses in glioblastoma cells. In summary, the preclinical evidence suggests that Mebendazole has potential as a therapeutic agent for brain cancer. It inhibits tumor cell growth, targets angiogenesis and Hh signaling, sensitizes cancer cells to conventional therapies, and shows promising results in preclinical models. 1
Clinical Evidence of Antitumoral Properties
Clinical evidence suggests that Mebendazole may possess antitumoral properties and could potentially be used as an adjuvant or neoadjuvant therapeutic for cancer treatments. Currently, there are six clinical trials registered at clinicaltrials.gov investigating the anticancer effect of Mebendazole, either alone or in combination with other drugs. The first clinical case report on Mebendazole as a cancer treatment in a human patient was published in 2011 by Dobrosotskaya et al. The study involved a 35-year-old woman affected by metastatic adrenocortical carcinoma who showed tumor progression after repeated surgeries, radiation, and chemotherapy treatments. After administering Mebendazole orally twice daily for 19 months, the researchers observed prolonged tumor response, as the liver metastases initially regressed and remained stable for 19 months. The study also demonstrated reduced angiogenesis in the tumor treated with Mebendazole, which may result from diminished tumor growth. Another study by Nyger and Larsson reported that Mebendazole induced remission of lung and lymph node metastases and a partial remission of liver metastases in a patient with refractory colon cancer. In this case, a 74-year-old patient affected by metastatic colon cancer in progression at multiple sites received Mebendazole monotherapy. After six weeks of treatment, the patient showed near-complete remission of the metastases in the lungs and lymph nodes and a good partial remission in the liver. A phase I clinical study completed in 2021 at John Hopkins Hospital investigated the maximum tolerated dose (MTD) of Mebendazole in combination with TMZ after surgery and standard radiation and TMZ treatment in patients with newly diagnosed malignant gliomas. The study supports that oral Mebendazole can be used safely in high doses in combination with TMZ. Two ongoing clinical trials investigate the anticancer effects of Mebendazole in combination with standard-of-care agents for recurrent pediatric brain cancers that are not responding to standard therapies. The primary objective of these trials is to determine if Mebendazole is tolerated when used in combination with the current three-drug regimen, and then evaluate the efficacy of this regimen after determining the maximally tolerated dose for each group. In conclusion, while further clinical evaluation of Mebendazole is still needed, the available clinical evidence suggests that Mebendazole may possess antitumoral properties and could potentially be used as an adjuvant or neoadjuvant therapeutic for cancer treatments. 2
Reference
1. Meco D, Attinà G, Mastrangelo S, Navarra P, Ruggiero A. Emerging Perspectives on the Antiparasitic Mebendazole as a Repurposed Drug for the Treatment of Brain Cancers. Int J Mol Sci. 2023;24(2):1334.
2. Chai JY, Jung BK, Hong SJ. Albendazole and Mebendazole as Anti-Parasitic and Anti-Cancer Agents: an Update. Korean J Parasitol. 2021;59(3):189-225.
);You may like
Related articles And Qustion
Lastest Price from Mebendazole manufacturers
US $0.00-0.00/kg2024-04-29
- CAS:
- 31431-39-7
- Min. Order:
- 1kg
- Purity:
- 98.0%
- Supply Ability:
- 1tons

US $0.00/kg2024-04-05
- CAS:
- 31431-39-7
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 1000kg