LOXO-292: A Promising RET Inhibitor with Strong Anti-Tumor Effects and Manageable Adverse Events
General Description
LOXO-292 is a potent inhibitor of the RET signaling pathway, demonstrating strong inhibition of cell proliferation in cancer cell lines with RET alterations, while exhibiting lower activity in cell lines without RET alterations. It has shown anti-tumor effects in preclinical models and induced tumor regression even in cases with acquired resistance mutations. Pharmacokinetic studies indicate dose-proportional increases in AUC and Cmax, with a steady-state achieved after approximately 7 days. Adverse events from clinical trials include common reactions such as dry mouth, diarrhea, and hypertension, as well as grade 3–4 adverse reactions and laboratory abnormalities. Serious adverse reactions occurred in 33% of patients, with 3% experiencing fatal events. These findings suggest that LOXO-292 selectively targets cancer cells with RET alterations and has the potential to be an effective treatment for patients with RET-driven cancers.

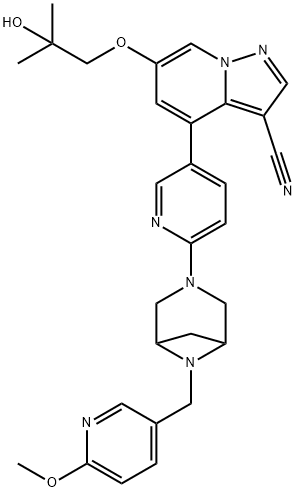
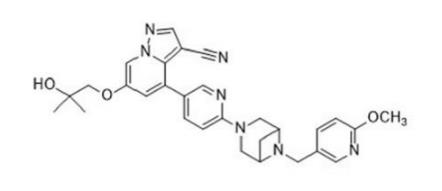
Figure 1. LOXO-292
Pharmacodynamics
LOXO-292 is a potent inhibitor of RET (rearranged during transfection) signaling pathway, which plays a crucial role in the development and progression of certain types of cancers. In laboratory studies, LOXO-292 demonstrated strong inhibition of cell proliferation in various cancer cell lines with RET alterations, including RET-mutant medullary thyroid carcinoma (MTC), RET fusion-positive thyroid papillary carcinoma, and non-small cell lung cancer (NSCLC) cells. However, it exhibited significantly lower inhibitory activity against cell lines without RET alterations. Furthermore, LOXO-292 has shown promising anti-tumor effects in preclinical models, both engineered and patient-derived, including those with acquired resistance mutations. It induced tumor regression in RET fusion-positive and RET-mutant murine models, even in cases with the V804M gatekeeper mutation associated with acquired resistance. In a mouse model of intracranial RET fusion-positive cancer, oral administration of LOXO-292 significantly prolonged survival compared to the control group. Additionally, the impact of LOXO-292 on the QTc interval, a measure of cardiac electrical activity, was evaluated in volunteers. The maximum mean increase in QTc interval was predicted to be 10.6 ms at steady-state peak drug concentration. These findings suggest that LOXO-292 selectively targets cancer cells with RET alterations, inhibits tumor growth, and has the potential to be an effective treatment option for patients with RET-driven cancers. 1
Pharmacokinetics
LOXO-292's pharmacokinetics in patients with cancer show dose-proportional increases in AUC and Cmax at steady-state between doses of 20 and 240 mg twice daily. It reaches steady-state after approximately 7 days with a 3.4-fold median accumulation ratio at a dose of 160 mg twice daily. The mean steady-state Cmax is 2980 ng/mL, AUC0-24h is 51600 ng·h/mL, and median tmax is 2 hours. LOXO-292 has a mean absolute bioavailability of 73% in volunteers and is not significantly affected by a high-fat meal. The drug has an apparent clearance (CL/F) of 6 L/h and a half-life (t½) of 32 hours. Metabolism primarily occurs through cytochrome P450 (CYP) 3A4. LOXO-292 is eliminated mainly through unchanged drug, with 69% and 24% recovered in feces and urine, respectively. Body weight affects the volume of distribution and clearance, while age, gender, and renal impairment do not significantly impact pharmacokinetics. Hepatic impairment increases exposure to LOXO-292, and drug interactions with CYP3A inhibitors and inducers can alter its pharmacokinetic profile. 2
Adverse events
Adverse events associated with LOXO-292 in the LIBRETTO-001 trial included a range of reactions. Common adverse reactions (grade 1–4) occurring in ≥25% of patients were dry mouth (39%), diarrhea (37%), constipation (25%), hypertension (35%), fatigue (35%), edema (33%), and rash (27%). Grades 3–4 adverse reactions occurring in ≥2% of patients included hypertension (18.0%) and dyspnea (2.3%), with grade 3 only adverse reactions such as diarrhea (3.4%), fatigue (2.0%), and prolonged QT interval (4%). Furthermore, laboratory abnormalities (≥25%) worsening from baseline in patients treated with LOXO-292 included increased AST levels (51%), increased ALT levels (45%), increased glucose levels (44%), decreased albumin levels (42%), decreased calcium levels (41%), increased creatinine levels (37%), increased alkaline phosphatase levels (36%), increased total cholesterol levels (31%), decreased sodium levels (27%), decreased leukocyte levels (43%), and decreased platelet levels (33%). Grade 3–4 laboratory abnormalities occurring in ≥2% of patients included increased AST levels (8%), increased ALT levels (9%), increased glucose levels (2.2%), decreased calcium levels (3.8%), increased alkaline phosphatase levels (2.3%), increased bilirubin (2.0%), and decreased platelets (2.7%). Additionally, serious adverse reactions were observed in 33% of patients, most frequently pneumonia. Fatal adverse reactions occurred in 3% of patients, including sepsis, cardiac arrest, and respiratory failure. Five percent of patients required permanent discontinuation of LOXO-292 therapy due to adverse reactions, with dosage interruptions and reductions also being common due to various adverse reactions. 3
Reference
1. Subbiah V, Velcheti V, Tuch BB, et al. Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol. 2018;29(8):1869-1876.
2. Eli Lilly. RETEVMOTM (selpercatinib): US prescribing information. 2020.
3. Markham A. Selpercatinib: First Approval. Drugs. 2020;80(11):1119-1124.
You may like
Related articles And Qustion
Lastest Price from LOXO-292 manufacturers
US $0.00-0.00/kg2025-04-21
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- >99.5% by HPLC
- Supply Ability:
- 100kg/month
US $2.00/kg2025-04-21
- CAS:
- 2152628-33-4
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 30KG/M