Isovaleraldehyde: properties, metabolism and safety
General Description
Isovaleraldehyde, a colorless liquid with a pungent odor, has the molecular formula C5H10O and is used in chemical synthesis. It's slightly water-soluble, highly miscible with organic solvents, and less dense than water. With boiling and melting points of 90°C and -60°C respectively, it's volatile and can contribute to smog. Metabolically, it's processed by oxidation to isovaleric acid, glutathione conjugation, and CYP450 enzyme reactions. It poses safety risks like irritation, flammability, and potential toxicity, requiring careful handling despite being an approved food flavoring agent.

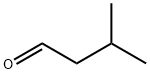
Figure 1. Isovaleraldehyde
Properties
Isovaleraldehyde is an organic compound with the molecular formula C5H10O. It is a colorless liquid at room temperature and has a strong pungent odor reminiscent of fermented apples or cheeses. This aldehyde is slightly soluble in water but highly miscible with most organic solvents, such as ethanol and ether. Isovaleraldehyde has a boiling point of approximately 90 degrees Celsius and a melting point of -60 degrees Celsius. Its density is about 0.8 g/cm³, which is less than that of water, indicating that it will float if mixed with aqueous solutions. The compound exhibits a moderate vapor pressure, suggesting that it can evaporate into the air at room temperature. As a volatile organic compound (VOC), Isovaleraldehyde can contribute to photochemical smog when released into the atmosphere. It is used as an intermediate in the manufacture of various chemicals, including pharmaceuticals, flavors, and fragrances. 1
Metabolism
Isovaleraldehyde is an aldehyde that undergoes metabolic processing in the body through various pathways. One primary route of metabolism for aldehydes like isovaleraldehyde is oxidation by the enzyme aldehyde dehydrogenase (ALDH), which converts them into their corresponding carboxylic acids. For isovaleraldehyde, this oxidation process results in the formation of isovaleric acid, a metabolite that can be further broken down through the fatty acid oxidation pathway or enter the Krebs cycle for energy production. Another detoxification pathway involves the conjugation of aldehydes with glutathione, a sulfhydryl-containing antioxidant. This reaction typically occurs under conditions where ALDH activity is inhibited or glutathione levels are depleted, leading to increased aldehyde toxicity. Isovaleraldehyde can also be metabolized by cytochrome P450 enzymes (CYP450), a family of enzymes responsible for the oxidative metabolism of various xenobiotics. These enzymes can catalyze the formation of olefinic products from aldehydes, particularly those with branching at the alpha carbon, such as isovaleraldehyde. The extent of this reaction varies with different P450 isozymes and is influenced by the structural characteristics of the aldehyde substrate. In summary, isovaleraldehyde metabolism involves oxidation to carboxylic acids by ALDH, conjugation with glutathione, and transformation by CYP450 enzymes, all of which contribute to its detoxification and clearance from the body. 2
Safety
Isovaleraldehyde is a chemical compound that presents several safety hazards. It can cause health issues upon inhalation, leading to symptoms such as chest discomfort, nausea, vomiting, and headaches. Direct contact with the liquid form can irritate the skin and eyes, and ingestion can irritate the mouth and stomach. It is highly flammable, with a lower flammable limit of 1.7% by volume and an upper limit of 6.8%, making it easily ignitable by heat, sparks, or flames. Its vapors may form explosive mixtures with air and are heavier than air, allowing them to spread along the ground and accumulate in low or confined areas, posing explosion hazards both indoors and outdoors. Containers holding Isovaleraldehyde may explode when heated. Additionally, it is a mild skin irritant and has been identified as a severe eye irritant based on animal studies. There are also concerns regarding its potential to cause liver impairment. Despite these hazards, Isovaleraldehyde is considered safe for use as a flavoring agent in food. When handling this substance, strict safety protocols should be followed to mitigate the associated risks. 3
Reference
1. Isovaleraldehyde. National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 11552.
2. 3-METHYLBUTANAL. Hazardous Substances Data Bank, Hazardous Substances DataBank Number: 628.
3. Isovaleraldehyde. Haz-Map, Information on Hazardous Chemicals and Occupational Diseases, CAS Number: 590-86-3.
You may like
Related articles And Qustion
See also
Lastest Price from Isovaleraldehyde manufacturers

US $0.00/KG2025-04-21
- CAS:
- 590-86-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 100 MT

US $56.00/KG2025-03-26
- CAS:
- 590-86-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 2000T