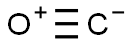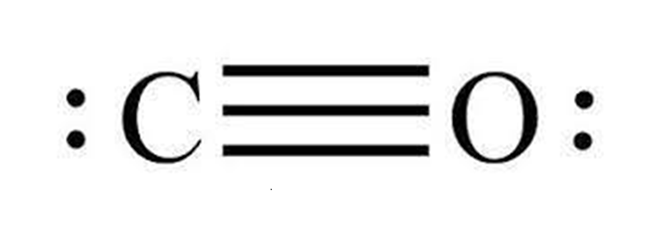Is Carbon Monoxide Polar or Nonpolar?
Polar Covalent Bonds
A bond in which the electronegativity difference between the atoms is between 0.5 and 2.0 is called a polar covalent bond. A polar covalent bond is a covalent bond in which the atoms have an unequal attraction for electrons and so the sharing is unequal. In a polar covalent bond, sometimes simply called a polar bond, the distribution of electrons around the molecule is no longer symmetrical. The ΔEN difference of 2.0 as the upper limit between polar covalent and ionic is arbitrary rather than an absolute cut off and that the properties of the compound are the best indicator of the primary nature of the bond. The differences in electronegativity are most valuable when used to predict the relative polarity of covalent bonds.
Polarity of Carbon Monoxide
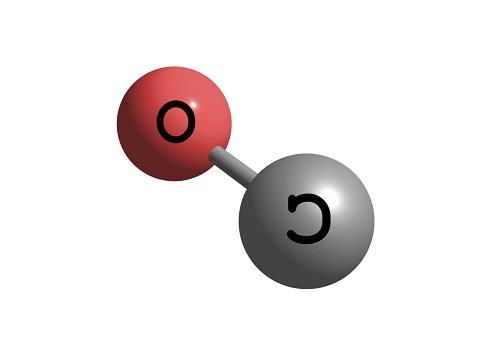
Carbon Monoxide or CO is a diatomic molecule. Carbon and Oxygen atoms share a triple bond to fill their octets. Both the atoms have lone pair of electrons and are sharing other electrons to complete their octets. It has a linear molecular geometry, given that there are only two atoms in this molecule. The arrangement of these lone pairs is symmetric, but for knowing if there is a dipole moment in the molecule, we need first to see the electronegativities of both the atoms and check if there is a difference between them.
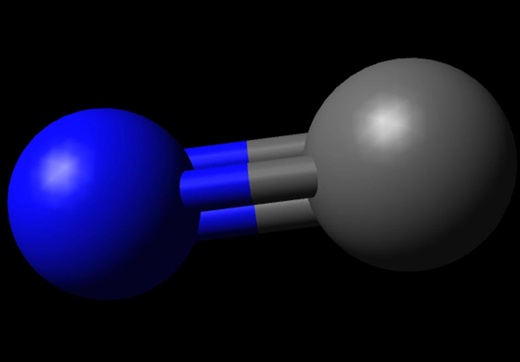
Carbon is a group 14 element on the periodic table having an electronegativity of 2.55, whereas Oxygen is a group 16 element having an electronegativity of 3.44.The oxygen atom is slightly higher in electronegativity, and hence it will try to pull the shared electrons to its side. This dipole moment in the molecule makes CO a polar molecule. The higher electronegativity if Oxygen makes it partially negatively charged and Carbon partially positively charged. And such formation of partial charges leads to the polarity in the molecule.
);