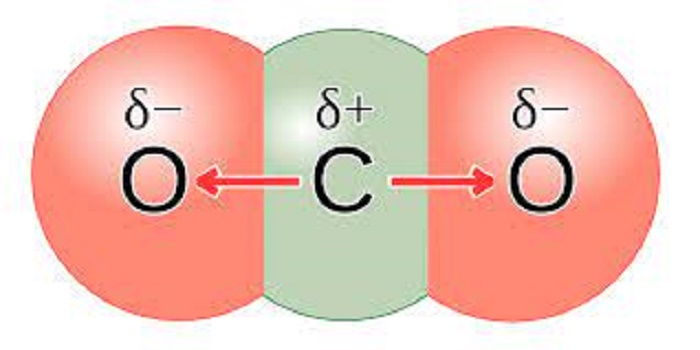
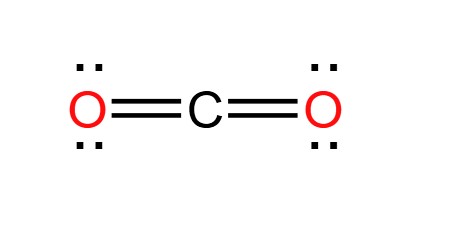
Is carbon dioxide a mixture or a pure substance?
Carbon dioxide (CO2) is indeed considered a pure substance.
A molecule of carbon dioxide (CO2) is made up of one carbon atom and two oxygen atoms. Even though it's composed of two types of elements, it's considered a pure substance because the composition is always the same - one part carbon to two parts oxygen.
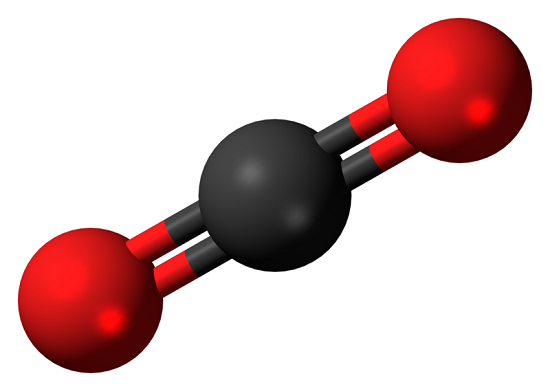
Carbon dioxide is a colorless and non-flammable gas at normal temperature and pressure. Although much less abundant than nitrogen and oxygen in Earth's atmosphere, carbon dioxide is an important constituent of our planet's air. It is also known as a greenhouse gas, that comes from the extraction and burning of fossil fuels (such as coal, oil, and natural gas), from wildfires, and natural processes like volcanic eruptions.
Uses
Carbon dioxide is used in the synthesis of urea, for organic synthesis, in the manufacture of dry ice and aspirin. It is also used in soft drinks, welding, fire extinguishers, and aerosol propellants.
CO2 is often used as a pesticide to store grains (at 60% concentration), respiratory stimulant, anesthetic, and euthanizing agent.
Industries that use carbon dioxide include fire extinguishing; processing, preserving, and freezing of food; metal working; livestock slaughtering; oil and gas recovery; and foundries.
It is also used to produce harmless smoke or fumes on a stage, chill golf ball centers before winding, and fumigate rice.
);