Idarubicin hydrochloride: Uses, Mechanism of action, Pharmacokinetics and Side effects
Uses of Idarubicin hydrochloride
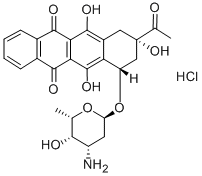
Idarubicin hydrochloride is an anthracycline antineoplastic drug and topoisomerase II inhibitor. Idarubicin hydrochloride is approved by the FDA for use in the treatment of acute myeloid leukaemia (AML) with other drugs. Idarubicin hydrochloride is also being studied for the treatment of other types of cancer.
IDAMYCIN, a sterile lyophilized powder for reconstitution and intravenous
administration, is available in a 20 mg single use only vial.
Each 20 mg vial contains 20 mg Idarubicin Hydrochloride, USP and 200 mg of Lactose
NF (hydrous) as an orange-red, lyophilized powder.
Mechanism of action
Idarubicin hydrochloride is a DNA-intercalating analog of daunorubicin which has an
inhibitory effect on nucleic acid synthesis and interacts with the enzyme topoisomerase
II. The absence of a methoxy group at position 4 of the anthracycline structure gives the
compound a high lipophilicity which results in an increased rate of cellular uptake
compared with other anthracyclines.
Pharmacokinetics
In 21 patients with acute leukemia, idarubicin hydrochloride, a new anthracycline antitumor drug, was administered i.v. for 3 consecutive days to study the pharmacokinetics. The terminal half-lives (t1/2) of the drug in these patients were 6.40-15.10 hrs. in plasma, and 8.09-16.34 hrs. in blood cells. Its main metabolite idarubicinol remained longer in blood; t1/2 values were 43.46-51.01 hrs. in plasma and 36.61-54.70 hrs. in blood cells. After 2-4 hrs, the concentrations of idarubicinol in both plasma and blood cells exceeded those of idarubicin. The AUCs of idarubicinol in plasma were 5.16-8.36 times higher than those of idarubicin, and AUCs of idarubicinol in blood cells were 2.05-4.57 times higher than those of idarubicin. Among the doses ranged from 5 to 15 mg/m2/day, the AUCs of both idarubicin and idarubicinol increased dose-dependently. In 2-compartment multiple dose models, plasma t1/2 alpha and t1/2 beta of idarubicin were 0.25 +/- 0.13 hrs. and 9.4 +/- 3.4 hrs., respectively. The steady-state volume of distribution (Vdss) was 934.9 +/- 370.7 l/m2, and the plasma clearance was 82.3 +/- 29.7 l/hr/m2. The urinary excretion of the drug was comparatively low. Until 7 days after administration, the mean cumulative urinary recovery rates of idarubicin and idarubicinol were 2.04% and 11.53%, respectively, and 13.57% in total.
Side effects
The adverse reactions that may occur with Idarubicin hydrochloride injection include: infections, nausea and vomiting, hair loss, abdominal pain/diarrhoea, bleeding, mucositis, skin disorders, fever, headache, mental or heart problems. Rarely, lung hypersensitivity, seizures, and cerebellar problems may be seen.
References:
[1] H FUJITA. [A pharmacokinetic study of idarubicin hydrochloride, a new anthracycline anti-tumor drug, in patients with acute leukemia. Idarubicin Study Group].[J]. Japanese Journal of Cancer and Chemotherapy, 1992.
[2] T MASAOKA. [A late phase II study of idarubicin hydrochloride in adult patients with acute lymphocytic leukemia. Idarubicin Study Group].[J]. Japanese Journal of Cancer and Chemotherapy, 1993.
You may like
See also
Lastest Price from Idarubicin hydrochloride manufacturers

US $0.00/KG2024-03-12
- CAS:
- 57852-57-0
- Min. Order:
- 1KG
- Purity:
- 98% HPLC
- Supply Ability:
- 5000kgs

US $0.00/g2023-11-15
- CAS:
- 57852-57-0
- Min. Order:
- 1g
- Purity:
- 99%min
- Supply Ability:
- 1000g