How to synthesize Glasdegib?
Description
Glasdegib was approved in 2018 for the treatment of newly diagnosed acute myeloid leukemia (AML) patients at least 75 years of age who are not appropriate candidates for intensive induction chemotherapy. The drug, developed by Pfizer, was approved for use with low doses of the anti-metabolite cytarabine. After receiving orphan drug designation for this indication in the U.S. and EU, glasdegib represents the first hedgehog (Hh) pathway inhibitor to be approved for AML in the U.S. Glasdegib also received orphan drug status in 2017 in the United States for treatment of myelodysplastic syndrome (MDS), and clinical trials remain ongoing for this and other hematological malignancies worldwide[1].
Clinical trials of previously untreated AML patients treated with glasdegib in combination with low doses of cytarabine showed significant reduction in risk of death and a 5-fold increased chance of obtaining complete remission versus those patients being treated with only cytabarine. Glasdegib binds to and inhibits Smoothened (SMO), a transmembrane protein of the same name, which is a component of the hedgehog pathway and is critical for disease progression.
Synthetic method
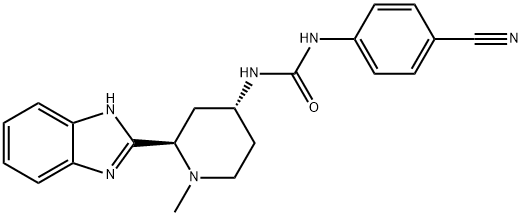
The scale synthesis of glasdegib has been described by researchers from Pfizer. It involves a kinetic resolution−amination approach, which is described below[2].
Synthesis of Glasdegib Piperidone 285
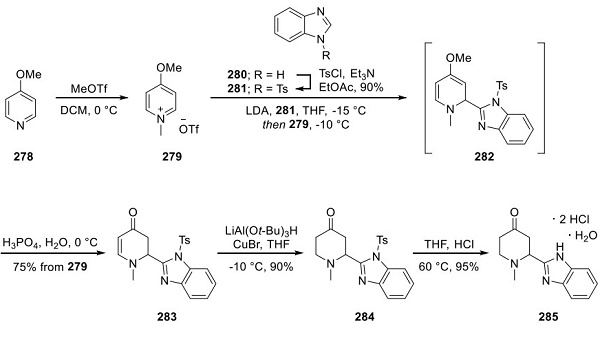
Methoxypyridine 278 was converted to the pyridinium salt 279 upon subjection to methyl triflate. Separately, benzimidazole 281, which arose from the tosylation of benzimidazole 280, was lithiated, quenched with 279, and then immediately treated with aqueous H3PO4 to yield enone 283 in 75% from 279. Subsequent 1,4-reduction with LiAl(OtBu)3 and CuBr provided piperidone 284 in high yield (90%) with only trace amounts (<2%) of the over-reduced saturated piperidinol observed. Tosyl removal with hot HCl in THF provided dihydrochloride monohydrate salt 285 in 95% yield as a racemate.
Transaminase Dynamic Kinetic Resolution (DKR) of Glasdegib Amine 286
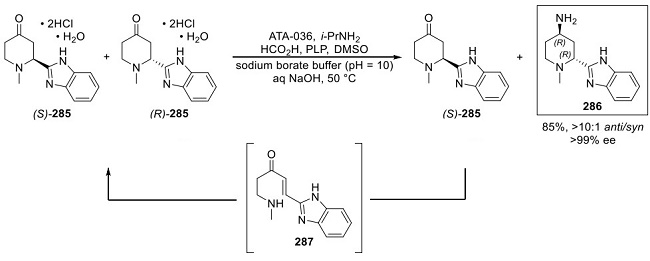
The key step of the synthesis relied on a dynamic kinetic resolution (DKR)-based enzymatic transamination process of the racemic mixture of (S)-285 and (R)-285 to provide the desired anti-amine 286 in high anti/syn ratio and high ee . As such, treatment of 285 in DMSO/borate buffer (pH = 10) with transaminase enzyme ATA-036 led to a>10:1 ratio of anti/syn amines 286 (99% ee). This transformation reportedly relied on the specificity of the transaminase reaction to proceed only with the (R)-285 isomer, with (S)- 285 instead undergoing a retro aza-Michael/aza-Michael racemization process (via 287) to regenerate ketones (S)- 285 and (R)-285 as a mixture with amine 286. This mixture is funneled back through the transamination process, ultimately providing amine 286 in 85% yield.
Synthesis of Glasdegib
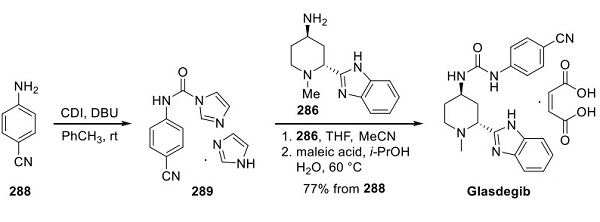
After completion of the transaminase DKR transformation, direct treatment of the reaction mixture with a solution of imidazole solvate 289 (prepared by reaction of aniline 288 with CDI and DBU as shown above) provided glasdegib in the free base form in 80% yield (98% purity) after precipitation from acetonitrile. This material could be converted directly to the corresponding maleate salt by stirring with maleic acid in aqueous i-PrOH at 60 °C, leading to a 95% isolated yield of glasdegib.
References
[1] Hoy, Sheridan M. “Glasdegib: First Global Approval.” Drugs 79 2 (2019): 207–213.
[2] Andrew C. Flick. “Synthetic Approaches to New Drugs Approved during 2018.” Journal of Medicinal Chemistry 63 19 (2020): 10652–10704.
);You may like
See also
US $0.00-0.00/g2023-05-08
- CAS:
- 1095173-27-5
- Min. Order:
- 100g
- Purity:
- 98%
- Supply Ability:
- kg grade