Health Hazard of Oxalic acid
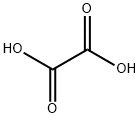
Oxalic acid is an organic acid with the IUPAC name ethanedioic acid and formula HO2C−CO2H. It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early investigators isolated oxalic acid from flowering plants of the genus Oxalis, commonly known as wood-sorrels. It occurs naturally in many foods, but excessive ingestion of oxalic acid or prolonged skin contact can be dangerous.
Oxalic acid has much greater acid strength than acetic acid. It is a reducing agent and its conjugate base, known as oxalate (C2O2−4), is a chelating agent for metal cations. Typically, oxalic acid occurs as the dihydrate with the formula C2H2O4·2H2O.

Applications
Oxalic acid's main applications include cleaning or bleaching, especially for the removal of rust (iron complexing agent). Its utility in rust removal agents is due to its forming a stable, water-soluble salt with ferric iron, ferrioxalate ion. The cleaning product Zud contains oxalic acid.Oxalic acid is an ingredient in some tooth whitening products. About 25% of produced oxalic acid will be used as a mordant in dyeing processes. It is also used in bleaches, especially for pulpwood, and for rust removal and other cleaning, in baking powder,and as a third reagent in silica analysis instruments.
Preparation of Oxalic acid
Oxalic acid can be easily prepared by oxidation of certain carbohydrates like sucrose by concentrates nitric acid. During oxidation, the carbon atoms are split off in pairs giving oxalic acid.
Health Hazard
Oxalic acid is a strong poison. The toxic symptoms from ingestion include vomiting, diarrhoea, and severe gastrointestinal disorder, renal damage, shock, convulsions, and coma. Death may result from cardiovascular collapse. Oxalic acid is an irritant of the eyes, mucous membranes, and skin. Inhalation or ingestion may result in kidney damage.
Oxalic acid is a strong poison. The toxic symptoms include renal damage, shock, convulsions. The toxicity arises as oxalic acid reacts with the calcium in the tissues to form calcium oxalate, thereby upsetting the calcium potassium ratio. The deposition of oxalates in the kidneys tubules may result in kidney damage.
Oxalic acid poisoning can result in headaches, dizziness, nausea, vomiting, convulsions, coma, and even death. A skin rash, discomfort, redness, blisters, and slow-healing ulcers can result from prolonged or recurrent exposure.
Toxicity
Oxalic acid has an oral LDLo (lowest published lethal dose) of 600 mg/kg.It has been reported that the lethal oral dose is 15 to 30 grams.The toxicity of oxalic acid is due to kidney failure caused by precipitation of solid calcium oxalate.
Oxalate is known to cause mitochondrial dysfunction.
Ingestion of ethylene glycol results in oxalic acid as a metabolite which can also cause acute kidney failure.
);You may like
Lastest Price from Oxalic acid manufacturers

US $15.00/kg2024-04-27
- CAS:
- 144-62-7
- Min. Order:
- 1kg
- Purity:
- 99.912%
- Supply Ability:
- 10ton

US $718.00-710.00/Tons2024-04-26
- CAS:
- 144-62-7
- Min. Order:
- 1Tons
- Purity:
- 99.99%
- Supply Ability:
- 100Tons