Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors
General Description
Fluorescein Sodium-guided surgery is a promising technique for improving the accuracy of tumor removal in neurosurgery. In addition to its use in glioma lesions, it has shown potential in non-glioma lesions such as cerebral metastases and skull base tumors. However, caution must be exercised due to potential adverse effects, and larger studies are needed to confirm its efficacy in non-glioma lesions. Overall, the use of Fluorescein Sodium has the potential to improve surgical outcomes and aid in the accurate identification and resection of brain tumors.

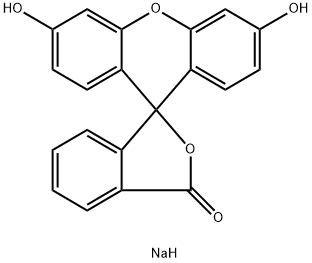
Figure 1. Fluorescein Sodium
Fluorescein Sodium in Modern Neurosurgery
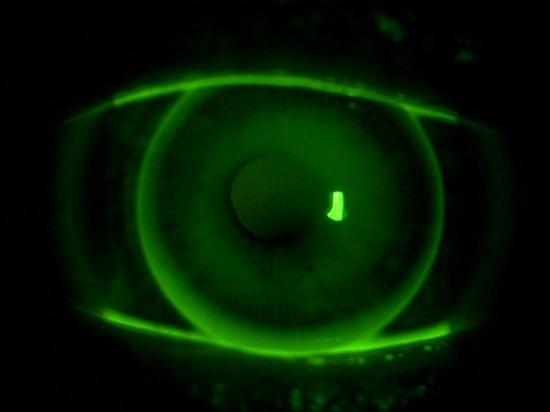
Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors is a technique that uses Fluorescein Sodium, an FDA-approved fluorescent dye, to highlight tumor tissue during surgery. The dye is injected into the patient at a high dose (15-20 mg/kg bodyweight) intravenously, and the surgeon views the tissue under white light illumination or a modified surgical microscope equipped with a blue light excitation filter and a barrier filter. The Fluorescein Sodium accumulates in areas where the blood-brain barrier is disrupted, making it easier for the surgeon to distinguish between normal tissue and tumor tissue. Several studies have shown that Fluorescein Sodium-guided surgery increases the rate of complete resection compared to non- Fluorescein Sodium-guided surgery. Additionally, the use of low-dose Fluorescein Sodium combined with dedicated filters has been shown to be effective in improving the quality of resection of malignant brain tumors. Fluorescein Sodium-guided surgery has the potential to increase the accuracy of tumor removal and improve patient outcomes without causing adverse effects. However, more research is needed to determine its long-term benefits and limitations. 1
Applications in Non-Glioma Lesions
The use of Fluorescein Sodium in non-glioma lesions has shown promising results in various types of brain tumors. In the case of cerebral metastases (CM), studies have demonstrated that Fluorescein Sodium-guided surgery can improve the rate of complete resection and potentially reduce recurrence rates compared to conventional surgery. Patients with CM who underwent microsurgery under white light after Fluorescein Sodium injection showed a high rate of complete resection and a low rate of local recurrence. Similar positive outcomes were observed in patients with CM when Fluorescein Sodium was used under specific filters, such as the Y560 filter. In addition to CM, Fluorescein Sodium has also been investigated in skull base tumors, including meningiomas. Fluorescein Sodium-guided surgery in these cases has allowed for clear visualization of dural enhancement, potentially indicating tumor infiltration. Furthermore, Fluorescein Sodium has shown promise in identifying small and deeply located primary central nervous system lymphoma (PCNSL) lesions. Although these findings are encouraging, larger prospective studies are needed to further confirm the efficacy and benefits of Fluorescein Sodium-guided surgery in non-glioma lesions. Nonetheless, the use of Fluorescein Sodium has the potential to improve surgical outcomes and aid in the accurate identification and resection of non-glioma brain tumors. 2
Considerations for Neurosurgeons
The use of Fluorescein Sodium in neurosurgery has been approved by the Italian Agency of Pharmacology (AIFA) but is considered off-label in other countries. Patients must provide written informed consent before undergoing Fluorescein Sodium-guided surgery. Though rare, severe adverse events have been reported in two cases involving high-dose Fluorescein Sodium injection during resection of high-grade gliomas. Both patients experienced severe hypotension during general anesthesia, and one patient had to discontinue surgery. However, both patients fully recovered after receiving adrenalin, prednisolone, and atropine. While there have been no reports of severe allergic reactions in the literature, it is important for neurosurgeons to be aware of potential liver and kidney dysfunction, pulmonary spasms, and history of allergies to contrast dye. In such cases, the use of Fluorescein Sodium should be reconsidered. It is worth noting that transient yellow discoloration of the skin and urine for up to 24 hours is a common, but harmless, side effect due to the rapid renal excretion of Fluorescein Sodium. Overall, while the use of Fluorescein Sodium has shown promising results, it is important for neurosurgeons to exercise caution and carefully consider the risks and benefits of its use in individual cases. 1
Reference
1. Schebesch KM, Brawanski A, Hohenberger C, Hohne J. Fluorescein Sodium-Guided Surgery of Malignant Brain Tumors: History, Current Concepts, and Future Project. Turk Neurosurg. 2016;26(2):185-194.
2. Da Silva CE, da Silva VD, da Silva JL: Sodium fluorescein in skull base meningiomas: A technical note. Clin Neurol Neurosurg. 2014;120:32-35.
You may like
Related articles And Qustion
See also
Lastest Price from Fluorescein Sodium manufacturers

US $0.00-0.00/KG2025-12-13
- CAS:
- 518-47-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $5.00-0.50/KG2025-06-13
- CAS:
- 518-47-8
- Min. Order:
- 0.10000000149011612KG
- Purity:
- 99% hplc
- Supply Ability:
- 5000kg