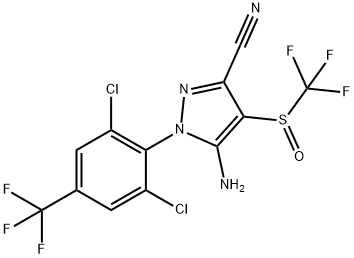
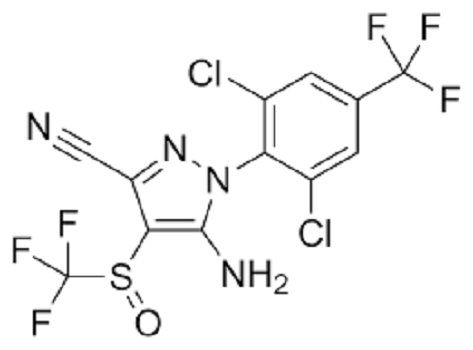
Fipronil: Toxicity, Applications, Preparation
Introduction
Fipronil is a broad-spectrum insecticide and acaricide that is widely used to control pests in crops, poultry, pets, and homes. It works by disrupting the function of the central nervous system in insects and mites, causing paralysis and death. Fipronil has been classified as a Group C (possible human) carcinogen by the US Environmental Protection Agency (EPA) based on animal studies, but the human carcinogenicity has not yet been fully evaluated. Fipronil is available in various formulations, including granules, dusts, liquids, and baits. It is commonly used to control termites, cockroaches, fleas, ticks, beetles, and other pests in residential and commercial settings. In agriculture, fipronil is used to control a wide range of insect pests in crops such as rice, corn, soybeans, cotton, fruits, and vegetables. While fipronil is generally considered safe for use in accordance with label instructions, improper application or exposure to high concentrations can pose risks to humans and other non-target animals. Fipronil can cause skin and eye irritation, headaches, dizziness, nausea, and vomiting. In pets, it can cause lethargy, diarrhea, and seizures. Therefore, it is important to follow recommended safety procedures and guidelines when using fipronil products[1].
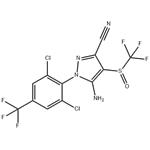
Figure 1 Appearance of Fipronil
Toxicity
Fipronil is considered moderately toxic for mammals, including humans, if exposure occurs through ingestion, inhalation, or absorption through the skin. The degree of toxicity depends on the concentration, duration, and route of exposure. Acute exposure to fipronil can cause symptoms such as nausea, dizziness, vomiting, abdominal pain, and headache. In severe cases, it can lead to seizures, respiratory depression, and even death. Long-term exposure to fipronil has been linked to kidney and liver damage, as well as effects on the reproductive and nervous systems. Fipronil is also highly toxic to bees and other pollinators, as well as fish and aquatic invertebrates. Fipronil contamination of water bodies has led to mass mortalities of fish and aquatic invertebrates in some instances. Due to its potential risks, fipronil is heavily regulated in many countries, and maximum residue limits (MRLs) have been established for fipronil residues in food and feed. It is important to follow recommended safety procedures and guidelines when using fipronil products, such as wearing protective clothing and gloves, avoiding inhalation and ingestion, and avoiding use near water sources or areas with sensitive non-target organisms.
It is also essential to properly store and dispose of fipronil and its formulations to prevent environmental contamination and minimize risks to human health and safety[2].
Applications
Fipronil is a highly effective broad-spectrum insecticide that has a wide range of applications in agriculture and veterinary medicine. In agriculture, it is commonly used to control pests such as beetles, grasshoppers, and weevils on crops like corn, rice, and cotton. Its potency against these insects makes it a valuable tool for farmers looking to protect their crops from damage and improve yields. In veterinary medicine, fipronil is often used to treat flea and tick infestations in dogs and cats. It is available in various formulations, including spot-on treatments, sprays, and shampoos, and can provide long-lasting protection against these parasites. While fipronil is known for its efficacy, it is important to use it according to label instructions and handle it with care due to its potential environmental and health risks.
It is important to note that fipronil should be used in accordance with label instructions and all relevant safety regulations and guidelines to minimize any potential risks to human health and the environment[3,4].
Preparation
Fipronil can be synthesized through a multi-step process that involves the functionalization of dichloromethane, followed by condensation, reduction, and amination reactions. Here is a brief overview of the synthesis of fipronil:
Step 1: Functionalization of Dichloromethane - The reaction between dichloromethane and sulfur results in the formation of dichloromethyl sulfide, which is then oxidized to form dichloromethyl sulfoxide. Dichloromethyl sulfoxide is further chlorinated to form dichloromethyl sulfone.
Step 2: Condensation - The dichloromethyl sulfone is then reacted with 5-amino-1-(2,6-dichloro-4-trifluoromethylphenyl)-4-trifluoromethylsulfonylpyrazole to form fipronil sulfone.
Step 3: Reduction - Fipronil sulfone is reduced using Raney nickel and hydrogen gas to give fipronil sulfoxide.
Step 4: Amination - Fipronil sulfoxide is then aminated using ammonia gas to produce the final product, fipronil.
After synthesis, fipronil is typically formulated into various commercial products such as sprays, baits, granules, and dusts for use in pest control and agriculture. It is important to handle fipronil and its formulations with care and follow all relevant safety protocols and guidelines[5].
References
[1] Stafford, C. A., Anderson, B. S., Lydy, M. J., et al. Fipronil: Ecotoxicology and Wildlife Toxicology [M]. In Ecotoxicology of Neurotoxicants. 2020, 347-391.
[2] Zhang, X., Yuan, S., Peng, W., et al. Fipronil: A Comprehensive Review of Recent Advances in Environmental Analysis and Degradation [J]. Water, Air & Soil Pollution. 2021, 232(2), 1-20.
[3] Meric, S. T., Kilic, A. E., & Karanfil, T. Preparation of Fipronil-Silver Nanoparticles as a Novel Pesticide Nanocomposite [J]. Journal of Agricultural and Food Chemistry. 2019, 67(3), 875-883.
[4] Singh, B., Singh, K. P., Agrawal, B., et al. Analysis, fate, and ecotoxicity of fipronil: A review [J] .Environmental Science and Pollution Research. 2020, 27(24), 29254-29270.
[5] Nasuti, C., Gabbianelli, R., Falcioni, G. Neurotoxicity and Neurobehavioral Effects of Fipronil [J] .Journal of Environmental Science and Health, Part B. 2017, 52(6), 402-408.
);You may like
Related articles And Qustion
Lastest Price from Fipronil manufacturers
US $10.00-2.00/KG2024-04-28
- CAS:
- 120068-37-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $1.00/KG2024-04-23
- CAS:
- 120068-37-3
- Min. Order:
- 1KG
- Purity:
- 99.91%
- Supply Ability:
- 200000