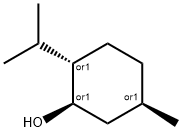
DL-Menthol: Natural Origin, Biosynthesis and Biological Properties
General Description
DL-Menthol, derived naturally from plants like peppermint, follows a complex biosynthesis pathway involving various enzymatic steps to produce its cooling and medicinal effects. With a natural origin preferred over synthetic forms due to purity concerns, DL-Menthol finds applications in pharmaceuticals, cosmetics, and food industries. Its biological properties, including analgesic, antibacterial, and antifungal effects, make it a versatile compound with potential therapeutic benefits. Research highlights its efficacy in pain relief, fungal inhibition, and antibacterial activity, positioning DL-Menthol as a valuable component in traditional medicine and a subject of interest for modern pharmacological studies.

Figure 1. DL-Menthol
Natural Origin
DL-Menthol is a naturally occurring compound primarily derived from aromatic plants, particularly through the steam distillation of cornmint oil. It belongs to the class of monoterpenes, which are organic compounds found in essential oils. These compounds, including DL-Menthol, serve various functions as chemical messengers in plants. The extraction process yields cornmint oil with a menthol content ranging from 55% to 85%. This natural origin is preferred over synthetic L-menthol due to concerns regarding the purity and scent of the latter. Synthetic L-menthol, created through a crystallization process, can be contaminated, affecting its scent negatively.DL-Menthol's natural origin ensures its authenticity and purity, making it desirable for various applications in industries such as pharmaceuticals, cosmetics, and food. Its distinct properties and aroma contribute to its widespread use in products ranging from topical analgesics to flavorings. 1
Biosynthesis
DL-Menthol, a compound known for its cooling sensation and medicinal properties, undergoes a complex biosynthesis pathway within plants. This biosynthesis occurs through an 8-step pathway from primary metabolism, involving specific enzymes and anatomical structures within the plant. The process begins with the formation of geranyl diphosphate, a universal monoterpene precursor, through the condensation of isopentenyl diphosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). This precursor is then transformed into limonene through cyclisation. Subsequently, limonene undergoes NADPH- and oxygen-dependent hydroxylation to form trans-isopiperitenol. Through allylic oxidation and NADPH-dependent reduction, isopiperitenone is converted into (+)-cis-isopulegone, which further undergoes isomerization to yield (+)-pulegone. (+)-Pulegone serves as the precursor for various compounds, including (+)-menthofuran, (+)-menthone, and (+)-isomenthone. Reduction of these ketones leads to the formation of DL-Menthol and its isomers, such as (+)-neomenthol, (+)-isomenthol, and (+)-neoisomenthol. This intricate biosynthesis pathway highlights the stepwise conversion of precursor molecules into DL-Menthol, showcasing the enzymatic processes and chemical transformations involved in its natural production within plants. Understanding DL-Menthol's biosynthesis pathway provides insights into its molecular structure and potential applications in pharmaceuticals, cosmetics, and food industries. 2
Biological Properties
DL-Menthol, a compound found abundantly in peppermint tea and peppermint essential oil, possesses a myriad of biological properties that have been extensively studied. These properties include analgesic, antibacterial, antifungal, anesthetic, penetration-enhancing, chemopreventive, and immunomodulating effects. The analgesic effect of DL-Menthol has been investigated in various studies. It has been found to alleviate hyperalgesia, a condition characterized by heightened sensitivity to pain stimuli. Research on healthy male volunteers showed that application of DL-Menthol resulted in a decrease in both cold- and heat-induced pain sensitivity over a period of time. Another study observed an increase in cold and warm pain detection thresholds after subjects consumed a lozenge containing DL-Menthol. Additionally, DL-Menthol was found to decrease cold pain and mechanical pain thresholds while increasing mechanical pain sensitivity, indicative of its efficacy against neuropathic pain. DL-Menthol also exhibits antifungal activity, inhibiting the growth of fungi such as Fusarium verticillioides and Rhizopus stolonifer. Studies have shown that DL-Menthol reduces fungal growth when applied in varying concentrations, with significant reductions observed even at relatively low concentrations. Furthermore, DL-Menthol demonstrates antibacterial properties, contributing to its traditional medicinal use in treating infections. While essential oils have historically been utilized for their antimicrobial activity, DL-Menthol's specific antibacterial effects have been less explored in isolation. Nevertheless, it is recognized as one of the active components contributing to the antimicrobial efficacy of peppermint oil. Overall, DL-Menthol's diverse biological properties make it a valuable compound in traditional medicine and a subject of interest in modern pharmacological research for its potential therapeutic applications. 2
Reference
1. Lawrence BM. The composition of commercially important mints. Mint the Genus Mentha. CRC Press, USA. 2006.
2. Kamatou GP, Vermaak I, Viljoen AM, Lawrence BM. Menthol: a simple monoterpene with remarkable biological properties. Phytochemistry. 2013;96: 15-25.
);You may like
See also
Lastest Price from DL-Menthol manufacturers
US $0.00-0.00/kg2024-06-12
- CAS:
- 89-78-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 500
US $0.00-0.00/kg2024-05-30
- CAS:
- 89-78-1
- Min. Order:
- 0.10000000149011612kg
- Purity:
- ≥99%
- Supply Ability:
- 20tons