Dimethylhexynediol: Properties, Production process and Uses
Dimethylhexynediol and dimethylhexanediol
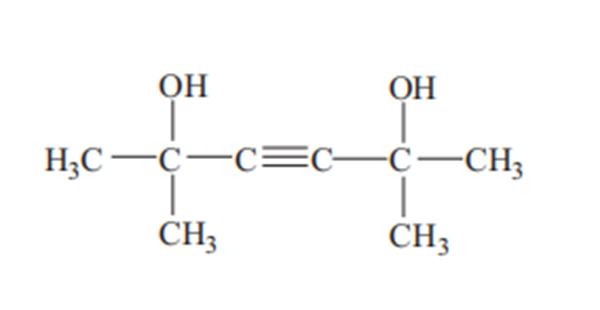
Dimethylhexynediol, also known as 2,5-dimethyl-3-hexyne-2,5-diol, 2,5-dimethylhexyne- 2,5-diol and tetramethylbutynediol, is a derivative of acetylene and acetone. CAS Registry Number: 142-30-3. Molecular formula: C8H14O2, molecular weight: 142.1956. The chemical structure is
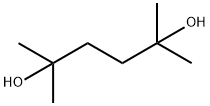
Dimethylhexanediol, also known as 2,5-dimethyl-2,5-hexanediol and 1,1,4,4-tetramethyl- 1,4-butanediol, is a derivative of dimethylhexynediol. CAS Registry Number: 110-03-2. Molecular formula: C8H18O2, molecular weight: 146.2273. The chemical structure is
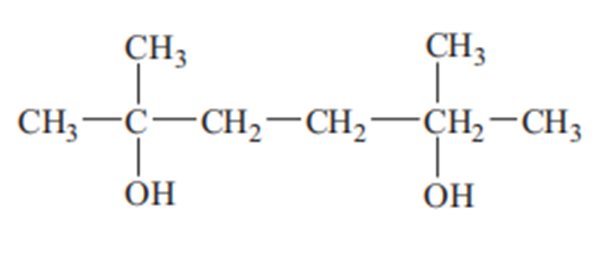
Properties of dimethylhexynediol and dimethylhexanediol
Dimethylhexynediol is a white crystal with a melting point of 94.5°C and a boiling point of 205.5°C. The vapor pressure is 0.9 kPa at 122°C. Dimethylhexanediol is also a white crystal with a melting point of 89°C and a boiling point of 214–215°C. The density of dimethylhexanediol is 0.898 at 20°C. Both dimethylhexynediol and dimethylhexanediol are soluble in water, acetone and alcohol, but they are insoluble in benzene, carbon tetrachloride and kerosene.
Production process of dimethylhexynediol and dimethylhexanediol
Dimethylhexynediol and dimethylhexanediol are mainly produced by ethynylation of
acetone (acetylene–acetone process) or by condensation of methylbutynol and acetone
(alkynol–acetone process).
(1) Manufacture of dimethylhexynediol by ethynylation of acetone
In the presence of potassium hydroxide catalyst, acetylene and acetone underwent a condensation reaction, then the reaction mixture was hydrolyzed and neutralized to obtain dimethylhexynediol:
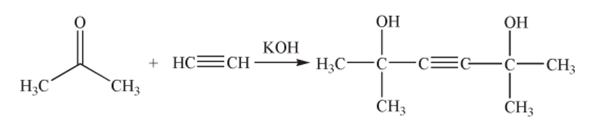
Finely divided and water-free potassium hydroxide powder was used as catalyst and suspended in organic solvents such as benzene or methyl tert-butyl ether. Acetone and acetylene were used in a molar ratio of 1:1 to 3:1 while KOH and acetone in a molar ratio of 1:1 to 1.6:1. The reaction was carried out at a temperature between 20 and 35 °C for 4 h. Subsequently, water was added to hydrolyze the reaction mixture for 1 h. The organic phase was separated and neutralized with glacial acetic acid. The conversion of acetone was 92% and the yield of dimethylhexynediol was 72%.
(2) Manufacture of dimethylhexynediol by condensation of methylbutynol
and acetone
Using methylbutynol and acetone as raw materials and xylene as a solvent, condensation reaction of methylbutynol with acetone is carried out in the presence of potassium isobutoxide catalyst to synthesize dimethylhexynediol. The chemical reaction equation is as follows:
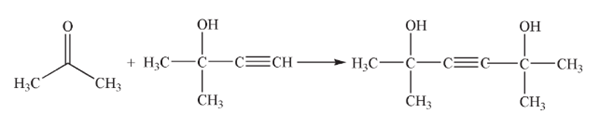
The synthesis process is carried out in two steps. First, isobutanol reacts with potassium hydroxide aqueous solution to form potassium isobutoxide, and then methylbutynol reacts with acetone to form dimethylhexynediol in the presence of potassium isobutoxide.
The preparation of potassium isobutoxide is a key step of the process. It is carried
out in a double-walled stirred vessel connected to a fractional distillation column.
Isobutanol and xylene are added to the vessel. An aqueous solution of potassium
hydroxide having a concentration of 33% by weight is introduced into the upper
section of the column. The temperature at the top of the column is about 91°C, and
the temperature at the bottom is 140°C. The azeotrope of water and isobutanol is
removed from the top of the column, condensed into liquid by cooling water and
separated into an organic layer and a water layer. A molar ratio of isobutanol and
potassium hydroxide in the range from 4.5:1 to 9:1 is applied in order to complete the
reaction. A suspension of potassium isobutoxide in xylene is obtained in the stirred
vessel. The conversion of potassium hydroxide is more than 99%.
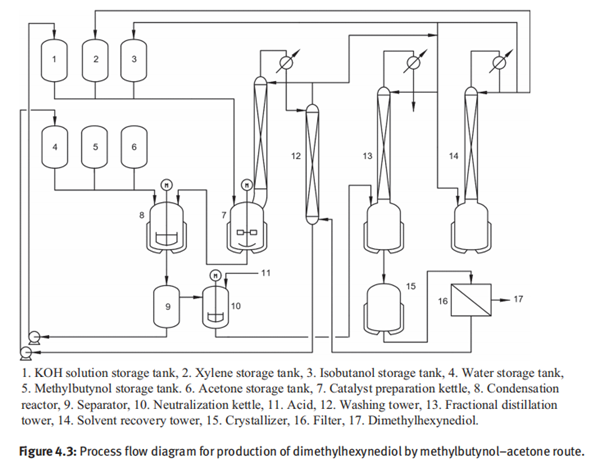
The suspension of potassium isobutoxide is cooled down to 0°C, and then equimolar acetone and methylbutynol were added. The suspended solids disappeared, and the entire reaction system became a homogeneous solution. The condensation reaction is carried out at 25–35°C for 3h. After the reaction is completed, water is added for hydrolyzation for 2h. The reaction mixture is allowed to stand for two layers, and an organicphase and an aqueous phase are separated. The aqueous phase contains potassium hydroxide and can be recycled for the preparation of potassium isobutoxide. The organic phase is neutralized with glacial acetic acid. Following partly removal of the solvent and cooling to room temperature, the dimethylhexynediol is obtained by crystallization. The overall yield of dimethylhexynediol is greater than 89%. Figure 4.3 shows the process flow diagram for production of dimethylhexynediol by methylbutynol–acetone route.
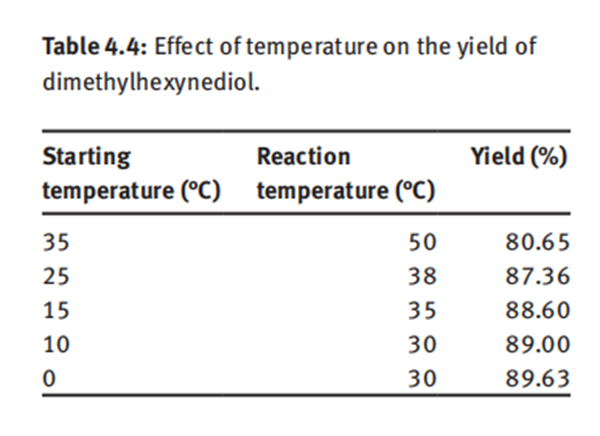
It is worth noting that the reaction is exothermic, and as the reaction temperature increases, the yield of dimethylhexynediol decreases (Table 4.4).
(3) Production of dimethylhexanediol by hydrogenation of dimethylhexynediol
The hydrogenation of dimethylhexynediol comprises two consecutive reactions. The first reaction is the partial saturation of the acetylenic bond to a double bond, leading to the generation of dimethylhexenediol. Subsequently, the dimethylhexenediol is further hydrogenated to form dimethylhexanediol:
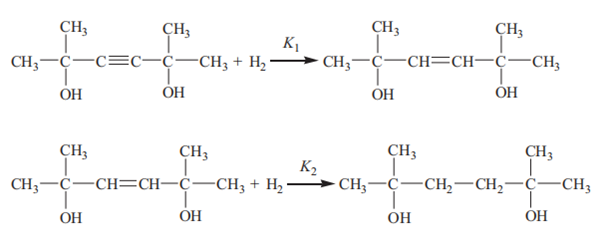
The reaction equilibrium constants K1 and K2 have been correlated as a function of temperature in the range from 300 to 345 K:
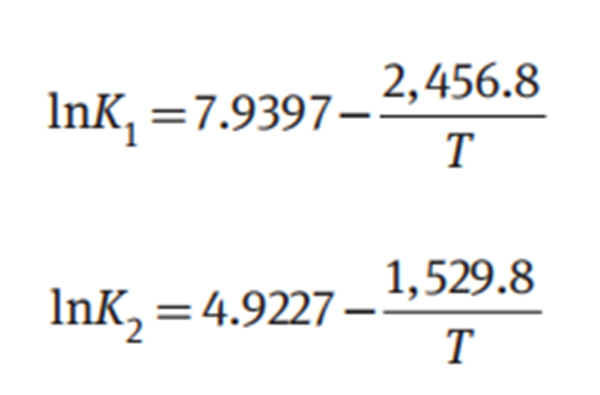
The hydrogenation process of dimethylhexynediol can be conducted in a batch or continuous operation. For a batch operation, dimethylhexynediol and solvent are charged to a suitable hydrogenation vessel containing catalyst such as palladium supported on activated carbon. Suitable solvents are benzene, ethylbenzene and water. Hydrogen is then introduced into the vessel under the desired pressure. The reaction proceeds in the temperature range from 30 to 70°C.
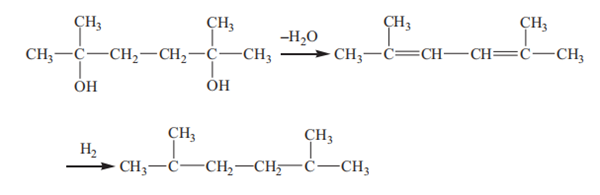
The hydrogenation process is conducted under relatively moderate temperatures. Generally, the temperature may range from about 30°C to about 80°C. If hydrogenation is carried out at too low a temperature, the time required for complete saturation is generally too long for commercial operation; and if the hydrogenation is conducted at too high a temperature, the production of the undesired hydrogenolysis products is favored. There are two side reactions in the reaction process: one is the dehydration reaction, which generates dialkene, and then the dialkene can be hydrogenated to alkane:
Another side reaction is the decomposition of dimethylhexynediol:
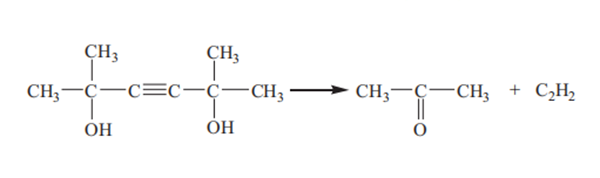
These side reactions occur at higher temperatures, leading to a decrease in the yield of dimethylhexanediol. The optimized reaction temperature is 60 °C.
Uses of methylhexynediol and dimethylhexanediol
Methylhexynediol can be used as a surfactant and as an intermediate in the synthesis
of herbicides. It is also used as a chelating agent for fiber and paper etchants, a metal
phosphate processing reagent, a lubricant for electric wires and an intermediate for
organic peroxides.
Dimethylhexanediol is an important raw material for high-efficiency and lowtoxic pyrethroid pesticides. It is also used as a combination of new plastic crosslinking agents, surfactants, artificial musk and fragrances.
);You may like
Lastest Price from 2,5-Dimethyl-2,5-hexanediol manufacturers

US $0.00/KG2024-05-22
- CAS:
- 110-03-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500mt

US $30.00/kg2024-05-22
- CAS:
- 110-03-2
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 2000kg