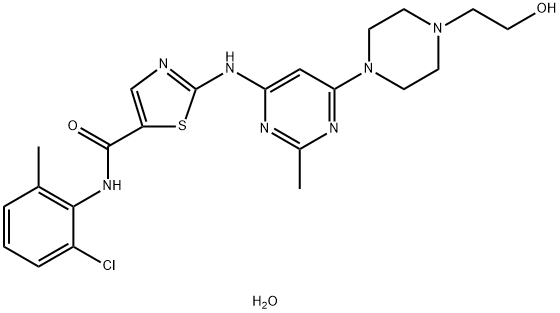
Dasatinib monohydrate: Structure and Synthesis method
Description
Dasatinib [N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole carboxamide monohydrate] is a potent, orally active, multitargeted inhibitor of several critical oncogenic kinases which was developed by Bristol-Myers Squibb. Dasatinib (DAS) was approved by the FDA in 2006 and is sold under the trade name Sprycel. DAS is a poorly water-soluble drug, and commercial DAS is a monohydrate (Dasatinib monohydrate) with a solubility of 8 μg/mL at 24 °C.
Structure
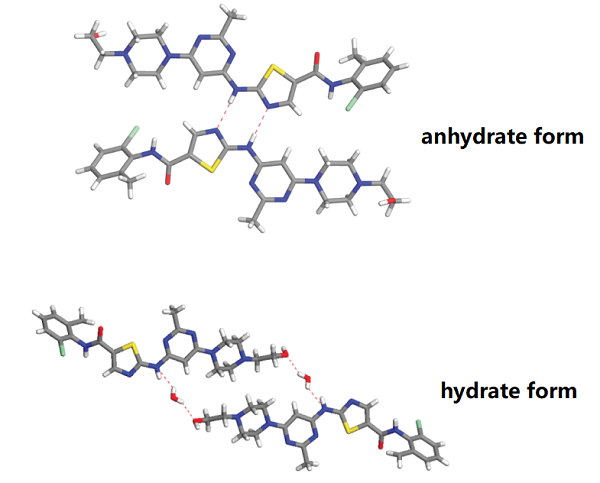
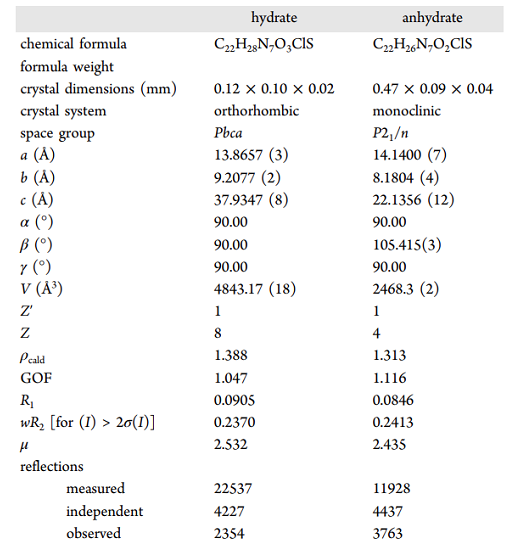
A better solubility than the commercial hydrate form characterizes an anhydrate phase of DAS. DAS is prone to solvate formation, and including solvents decreases the crystal's void space. Growing suitable single crystals of DAS for structure determination proved challenging due to the propensity of both anhydrate and hydrate phases to form small crystals with extensive twinning and modest crystal quality; despite these challenges, Roy et al. were able to determine the crystal structure for both hydrate and anhydrate phases for the first time. DAS anhydrate crystallizes in the monoclinic crystal system and is solved in the space group P21/n, whereas the hydrate phase is orthorhombic in Pbca (Table 1). DAS hydrate and anhydrate phases were detected in the 2800 to 3300 cm−1 region, and C−H vibrations from the aromatic ring and O−H stretching were observed[1].
Synthesis method
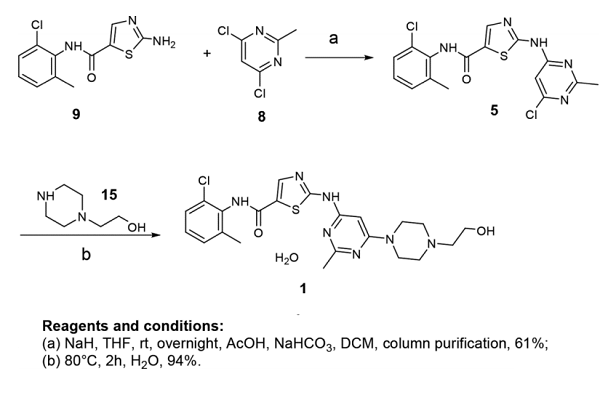
Jagabandhu Das and his co-workers reported the synthesis of Dasatinib monohydrate, as shown below. The initial step of synthesis involves the nucleophilic coupling of amino thiazole carboxamide 9 with 4,6-dichloro-2-methylpyrimidine 8 to give penultimate intermediate 5 in 61% yield, which was coupled with 2-(piperazin-1-yl)ethan1-ol (HEP) 15 and subsequent water addition to give final product Dasatinib monohydrate[2].
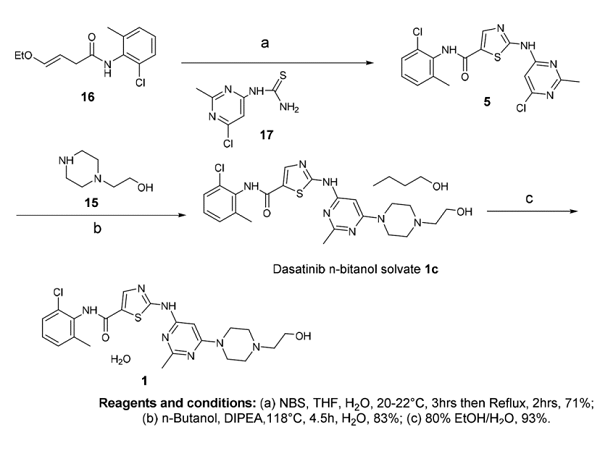
Chen, Bang-Chi et al. reported the synthesis of Dasatinib monohydrate as depicted below. The first step of the synthesis involves the α-bromination of βethoxyacrylamide 16 followed by coupling with thiourea compound 17 to give compound 5 which was condensed with HEP 15 in n-butanol gives a pseudo polymorph crystalline Dasatinib n-butanol solvate 1c. Further, compound 1c was used on treatment with 80% aqueous ethanol at 75°C, yielding the compound Dasatinib monohydrate. The overall yield of this route is 59% over 3 stages.
References
[1] Garbapu Suresh . “A convenient new and efficient commercial synthetic route for dasatinib (Sprycel®).” Synthetic Communications 47 17 (2017): Pages 1610-1621.
[2] Saikat Roy, Adam J. Matzger*, Rosalynn Quiñones. “Structural and Physicochemical Aspects of Dasatinib Hydrate and Anhydrate Phases.” Crystal Growth & Design 12 4 (2012): 2122–2126.
);You may like
Lastest Price from Dasatinib monohydrate manufacturers
US $32.00-1.30/KG2024-04-15
- CAS:
- 863127-77-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/kg2024-04-13
- CAS:
- 863127-77-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000kg