D-Camphor: Sources, Biosynthesis and Antimicrobial Activity
General Description
D-Camphor is a waxy, aromatic solid that sublimates at room temperature and melts at 180°C. It exists in two enantiomeric forms and is primarily derived from α-pinene found in turpentine oil. Natural D-Camphor is distilled from various trees, including the camphor laurel tree, the Borneo camphor tree, and the East African camphorwood tree. It is also found in camphor basil and various aromatic plant species. The biosynthesis of D-Camphor involves enzymatic processes within plants, starting with the biotransformation of geranyl diphosphate. Synthetic D-Camphor production utilizes turpentine oil and involves a series of chemical reactions. D-Camphor exhibits weak antimicrobial activity on its own but can enhance the antimicrobial effects of other compounds. It has been identified as a principal antimicrobial component in oils derived from plants like tea bush and rosemary. Overall, understanding the diverse sources, properties, and antimicrobial activity of D-Camphor is crucial for its utilization in various industries.

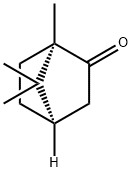
Figure 1. D-Camphor
Sources
D-Camphor, a waxy, aromatic solid, sublimates at room temperature and melts at 180°C, with limited solubility in water but soluble in various organic solvents. Its chemical formula is C10H16O, existing in two enantiomeric forms: (1S)-(−)- and (1R)-(+)-camphor. Although both forms share a camphoraceous odor, the impact of stereochemistry on biological activity remains uncertain. Synthetic D-Camphor is primarily derived from α-pinene found in turpentine oil, while natural D-Camphor, specifically (+)-camphor, is distilled from the wood of the camphor laurel tree, notably in Borneo and Taiwan, as well as from the Borneo camphor tree and the East African camphorwood tree. Additionally, camphor basil, particularly in Asia, serves as a significant source. Moreover, D-Camphor is a major component of essential oils in various aromatic plant species. Understanding its diverse sources and properties is crucial for its utilization in various industries. 1
Biosynthesis
The biosynthesis of D-Camphor involves intricate enzymatic processes within plants. It begins with the biotransformation of geranyl diphosphate (GPP), the preferred substrate, through cyclization by (+)-bornyl diphosphate synthase, yielding (+)-bornyl diphosphate. This compound is then hydrolyzed by bornyl-diphosphate diphosphatase to (+)-borneol. The final step involves the oxidation of (+)-borneol to D-Camphor catalyzed by (+)-borneol dehydrogenase. On the other hand, synthetic D-Camphor production utilizes turpentine oil as a starting material. α-Pinene, obtained from turpentine oil via distillation, undergoes a series of chemical reactions. Initially, α-pinene is converted to camphene through catalysis with a strong acid, using acetic acid as the solvent. Subsequently, camphene undergoes Wagner-Meerwein rearrangement to form the isobornyl cation, which then reacts with acetate to form isobornyl acetate. Hydrolysis of isobornyl acetate yields isoborneol, which is finally dehydrogenated to D-Camphor. It's noteworthy that the synthetic route from α-pinene results in a racemic mixture of D-Camphor, containing equal proportions of (−) and (+)-camphor. Understanding both the biosynthetic and synthetic pathways is essential for the production and utilization of D-Camphor in various industries. 2
Antimicrobial Activity
D-Camphor, a compound found in various essential oils, has been studied for its antimicrobial activity. Research has shown that camphor alone exhibits weak antimicrobial properties. For instance, essential oils containing D-Camphor as a major component, such as Greek sage oil, displayed poor activity against bacteria like Escherichia coli and Staphylococcus aureus. However, studies have also revealed that D-Camphor can enhance the antimicrobial effects of other compounds. When combined with 1,8-cineole, another essential oil component, camphor showed a synergistic effect, resulting in a significant reduction in colony forming units of microorganisms like Candida albicans. Additionally, D-Camphor has been identified as one of the principal antimicrobial components in oils derived from plants like tea bush and rosemary. In experiments investigating the antibacterial properties of rosemary oil, which contains a significant amount of camphor, it demonstrated effectiveness against various Gram-negative and Gram-positive bacteria responsible for meat spoilage. While D-Camphor alone may not exhibit strong antimicrobial activity against certain organisms, its presence in essential oil blends can contribute to overall effectiveness, suggesting a potential synergistic role with other active compounds. 2
Reference
1. Rebound Health. Camphor. 2013.
2. Chen W, Vermaak I, Viljoen A. Camphor--a fumigant during the Black Death and a coveted fragrant wood in ancient Egypt and Babylon--a review. Molecules. 2013; 18(5): 5434-5454.
);You may like
Related articles And Qustion
Lastest Price from D-CAMPHOR manufacturers

US $0.00/KG2024-06-03
- CAS:
- 464-49-3
- Min. Order:
- 1KG
- Purity:
- ≥98% HPLC
- Supply Ability:
- 1000KG

US $0.00-0.00/KG2024-05-28
- CAS:
- 464-49-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt