Azilsartan: an angiotensin receptor blocker for treatment of hypertension
General Description
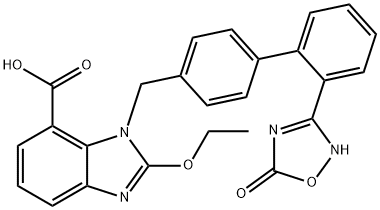
Azilsartan is a medication used to treat hypertension and has been shown to exhibit more potent antihypertensive action than other drugs in its class. Clinical trials have highlighted significant improvements in systolic and diastolic blood pressure, as well as 24-hour ambulatory blood pressure. Azilsartan has also been found to be well-tolerated and more efficacious at maximal dose than other medications in its class. Compared to other ARBs, azilsartan therapy resulted in greater reduction in blood pressure. Azilsartan-based combination therapy appears to be effective in controlling blood pressure regardless of race, and has a potential advantage over other ARB-based regimens in treating hypertension. However, it is important to note that side effects such as dizziness, increased serum creatinine, fatigue, diarrhea, hypotension, and syncope have been reported in clinical studies. As with any medication, caution should be exercised when prescribing azilsartan.
Figure 1. Tablets of azilsartan
![Article illustration]()
![Article illustration]() Mechanism of action
Mechanism of action
Azilsartan medoxomil is a prodrug which is hydrolysed in the gastrointestinal tract before getting absorbed in the system. Azilsartan acts against angiotensin II in a dose dependent manner. After administration of azilsartan to healthy subjects, plasma angiotensin I and II concentrations increased, while plasma renin activity increased while plasma aldosterone concentrations decreased. Azilsartan does not cause any clinical significant effects on serum sodium or potassium. 1
Clinical applications
Azilsartan medoxomil has been developed by Takeda Global Research & Development Centre, Inc., U.S. and got FDA approval in February 2011 for treatment in hypertension in adults. Azilsartan is now worldwide approved for hypertension either as a prodrug (Azilsartan medoxomil) or primary compound. Clinical trials have shown that it exhibits more potent antihypertensive action than other drugs in its class, with significant improvements in systolic and diastolic blood pressure as well as 24-hour ambulatory blood pressure. In addition, azilsartan has been found to be well-tolerated and more efficacious at its maximal dose than other medications in its class. A meta-analysis of randomized-controlled trials showed that azilsartan therapy resulted in greater reduction in blood pressure. 2, 3
Advantages
Azilsartan is closely related to candesartan with greater potency and prolonged duration of action as compared with other ARBs. Unlike candesartan which must be orally administrated as the prodrug (candesartan cilexetil) for better bioavailability, azilsartan is equally effective as either ester prodrug (azilsartan medoxomil) or primary compound itself. Azilsartan contains an oxo-oxadiazole ring which is not found in any of clinically approved ARBs, which makes azilsartan less acidic and more lipophilic than others. 2
Side effect
The side effects of Azilsartan, observed in clinical studies, include dizziness, increased serum creatinine, fatigue, diarrhea, hypotension, and syncope. Hypotension was the most common cause of drug discontinuation with monotherapy, while raised serum creatinine and dizziness were more prevalent when combined with chlorthalidone. Other reported side effects include muscle spasms, nausea, and hemogram abnormalities. Transient increases in serum creatinine were related to a significant drop in blood pressure and were exacerbated by old age and coadministration of diuretics. The drug's manufacturers also warn against administering this drug during pregnancy and to individuals who are volume or salt-depleted. 3
Combination therapy
There are several studies comparing azilsartan-based combination therapies, with most using azilsartan in combination with chlorthalidone. In the largest study by Cushman et al, azilsartan (40/80 mg) and chlorthalidone were compared with olmesartan (40 mg) and hydrochlorothiazide combinations in 1071 patients with stage 2 hypertension. Azilsartan-based combination regimens showed significantly better systolic BP lowering effects compared to olmesartan-based regimens. Similarly, Bakris et al demonstrated that an azilsartan plus chlorthalidone regimen resulted in greater fall in clinical systolic BP than azilsartan with hydrochlorothiazide in 609 patients with moderate-to-severe hypertension. The effectiveness of the blood pressure-lowering effect was maintained across both white and black races, as shown by Ferdinand et al in a pooled analysis from two RCTs. Therefore, azilsartan-based combination therapy appears to be effective in controlling BP, regardless of race, and has a potential advantage over other ARB-based regimens in treating hypertension. 2
Reference
1. Perry CM. Azilsartan medoxomil: a review of its use in hypertension. Clin Drug Investig, 2012, 32(9):621-639.
2. Pradhan A, Tiwari A, Sethi R. Azilsartan: Current Evidence and Perspectives in Management of Hypertension. Int J Hypertens, 2019, 2019:1824621.
3. Edarbi (Azilsartan Medoxomil), Prescribing Information, Arbor pharmaceuticals LLC, Atlanta, GA, USA, 2016.
);You may like
Related articles And Qustion
See also
Lastest Price from Azilsartan manufacturers

US $0.00/KG2024-04-28
- CAS:
- 147403-03-0
- Min. Order:
- 2KG
- Purity:
- 99% up / JP / GMP
- Supply Ability:
- 20tons

US $0.00-0.00/KG2024-04-25
- CAS:
- 147403-03-0
- Min. Order:
- 1KG
- Purity:
- 99
- Supply Ability:
- 50000KG/month